Received 24 October 2005 | Accepted 26 December 2005 | Published 28 December 2005
Reprint
Received 24 October 2005 | Accepted 26 December 2005 | Published 28 December 2005 |
Download Reprint |
Fluoxetine inhibits calcium-activated currents of salamander rod photoreceptor somata and presynaptic terminals via modulation of intracellular calcium dynamics
Ernest C. Steele, Jr., Xiaoming Chen,
Peter R. MacLeish
Neuroscience Institute, Department of Anatomy and Neurobiology, Morehouse School of Medicine, Atlanta, GA
Correspondence to: Dr. Ernest C. Steele, Jr., Neuroscience Institute, Department of Anatomy and Neurobiology, Morehouse School of Medicine, MRC Building, Room 220, 720 Westview Drive, SW, Atlanta, GA, 30310; Phone: (404) 756-6698; FAX: (404) 752-1041; email: esteele@msm.edu
Abstract
Purpose: In order to isolate voltage-gated calcium currents in rods retaining intact axons and presynaptic terminals, it is first necessary to identify specific blockers of the large calcium-dependent chloride current, ICl(Ca), which obscures them. Based upon previous reports of its efficacy as an inhibitor of a volume regulated chloride channel (VRAC), a calcium-dependent chloride channel, and the cystic fibrosis transmembrane conductance regulator (CFTR), we investigated whether the serotonin reuptake inhibitor, fluoxetine hydrochloride, could act as a specific blocker for ICl(Ca) in salamander rod photoreceptor terminals, without affecting other aspects of rod physiology.
Methods: Intact rod photoreceptors retaining axons and presynaptic terminals were enzymatically dissociated from salamander retinae. Under whole cell voltage clamp, depolarization-induced whole cell currents were recorded in the presence and absence of fluoxetine (10 and 50 μM pipette concentration) administered via a puffing pipette or in the bath (25 μM). Changes in intracellular free calcium levels were monitored as changes in fura-2 fluorescence following brief depolarization with high K+ (50 and 100 mM) administered via a puffing pipette in the presence and absence of fluoxetine (4 and 10 μM) in the bath.
Results: When puffed onto cells, fluoxetine inhibited ICl(Ca) in a dose-dependent fashion (50 μM=96% reduction; 10 μM=14% reduction). In addition to the reduction in amplitude of ICl(Ca), 4 μM fluoxetine (pipette concentration) significantly reduced the duration of ICl(Ca) (48% reduction). Fluoxetine also suppressed the calcium-activated potassium current, IK(Ca), to similar extents (50 μM=75% reduction; 10 μM=23% reduction) when puffed onto cells. Preincubation of rods with 25 μM fluoxetine in the bath significantly reduced outward currents at both 0 mV, where ICl(Ca) is negligible because ECl is about 0 mV and the bulk of the current is carried by IK(Ca), and at +40 mV, where the current is a combination of ICl(Ca) and IK(Ca). Parallel calcium imaging experiments with fura-2 revealed that preincubation of rods in 10 μM fluoxetine virtually eliminated the normal rise in intracellular free calcium in somatic (99.6% reduction) and terminal (98% reduction) compartments following brief depolarization with high K+ (100 mM pipette concentration). Cells preincubated in 4 μM fluoxetine, a therapeutically relevant concentration, showed smaller but significant reductions in Ca2+ elevations in both somatic (66% reduction) and terminal (36% reduction) compartments and even more significant reductions in the duration of sustained calcium levels of the terminal compartment (50% reduction) following brief depolarization with high K+ (50 mM pipette concentration).
Conclusions: We conclude that in addition to blocking ICl(Ca), fluoxetine inhibits IK(Ca). We further conclude that the inhibition of both of these currents is the consequence of inhibition of the normal sustained elevation in intracellular calcium following depolarization and initial calcium influx. Combined, the data suggest that fluoxetine may have multiple sites of action in rod photoreceptors instead of acting as a specific inhibitor of ICl(Ca).
Introduction
To date, most studies of calcium dynamics in rod photoreceptors have utilized isolated cells lacking intact axons and presynaptic terminals. Our laboratory has an interest in the local calcium dynamics of the terminal compartment, which we believe are independent, to a large extent, from somatic calcium dynamics. One obstacle encountered when trying to isolate calcium currents from rods retaining axons and presynaptic terminals, however, is a large inward calcium-dependent chloride current, ICl(Ca), which appears to be functionally localized to the terminal compartment [1] and is difficult to resolve from the voltage-gated calcium currents. Cells that have lost their axons and terminals during dissociation do not display significant calcium-dependent chloride currents, allowing for easy isolation of the somatic voltage-gated calcium currents. In order to isolate voltage-gated calcium currents in rods retaining intact axons and presynaptic terminals, it is first necessary to identify specific blockers of the large calcium-dependent chloride current, ICl(Ca), which obscures them. Fluoxetine hydrochloride, which was originally characterized as a specific serotonin reuptake inhibitor and is in common use today as an antidepressant therapeutic agent based upon this pharmacological property, has also been reported to inhibit a volume-regulated chloride channel (VRAC), a calcium-dependent chloride channel, and the cystic fibrosis transmembrane conductance regulator (CFTR) [2].
In the present study, we investigated the pharmacologic effects of fluoxetine hydrochloride on ICl(Ca) in enzymatically dissociated salamander rod photoreceptors retaining axons and presynaptic terminals to determine if fluoxetine could block ICl(Ca) without disturbing other parameters of rod physiology. Whole cell voltage clamp studies demonstrated an inhibition of both ICl(Ca) and a calcium-activated K+ current, IK(Ca), by fluoxetine. Parallel calcium imaging studies using fura-2 provide compelling evidence that the inhibitory effects of fluoxetine on these currents may actually be the consequence of a reduction in the normal sustained elevation in intracellular calcium following depolarization, presumably via inhibition of calcium influx through voltage-gated calcium channels. The calcium imaging data further demonstrate that this effect of fluoxetine on intracellular calcium dynamics is observed in both somatic and presynaptic terminal compartments, suggesting modulation of both signal forwarding through the soma and neurotransmitter release from the terminal by fluoxetine. To our knowledge, this work represents the first report of measured effects of fluoxetine on ionic currents and intracellular calcium dynamics associated with normal functioning of rod photoreceptors. Portions of these data have been previously published in abstract form [3].
Methods
Chemicals
Fluoxetine hydrochloride (+)-N-Methyl-γ-[4-(trifluoro-methyl)phenoxy]-benzenepropan-amine hydrochloride; LY110140 hydrochloride (Prozac) was purchased from Research Biochemicals International (Natick, MA). Fluoxetine was dissolved into DMSO to prepare a 10 mM stock solution. This was diluted into the saline solution described below to prepare working dilutions. Unless otherwise noted, all other chemical reagents were obtained from Sigma-Aldrich (St. Louis, MO).
Animals
Neotenic tiger salamanders, Ambystoma tigrinum (Kons Scientific Supply, Germantown, WI and Charles Sullivan, Inc., Nashville, TN) were maintained at 14 °C in a humidified incubator on a 12 h light and dark cycle. Animals were euthanized by decapitation and pithing prior to removal of eyes. All animal procedures conformed to the humane treatment of animals as prescribed by the Institute for Laboratory Animal Research (Guide for the Care and Use of Laboratory Animals) and by the Association for Research in Vision and Ophthalmology (ARVO) and were approved by the Morehouse School of Medicine Institutional Animal Care and Use Committee.
Dissociation and culture of retinal cells
Whole retinae were digested with 10 units/ml of papain (Worthington Biochemical, Lakewood, NJ) for 30 min at 25 °C in a salt solution (pH 7.2) bubbled for 10 min with 95% O2/5% CO2 and composed of the following (in mM): 85 NaCl, 25 NaHCO3, 1 Na pyruvate, 3 KCl, 0.5 NaH2PO4, 0.5 CaCl2, 1 cysteine, 0.02 phenol red, and 16 glucose. The tissue was then rinsed twice with 10 ml of a salt solution (pH 7.2) containing (in mM): 108 NaCl, 2 HEPES, 1 Na pyruvate, 0.5 MgCl2, 0.5 MgSO4, 0.5 NaH2PO4, 1.8 CaCl2, 3 KCl, 1 NaHCO3, 0.1 choline chloride, 0.02 phenol red, 16 glucose. The retinae were resuspended in another 10 ml of medium supplemented with an additional 16 mM glucose to increase density and then triturated in approximately 2 ml of this solution to yield single cells. Dispersed cells were plated onto modified tissue culture dishes in which the bottom plastic was replaced with glass coverslips pretreated with goat antimouse IgG secondary antibody and Sal-1 primary antibody [4]. BSA was added to medium at a final concentration of 50 μg/ml and the cells were incubated in a humidified incubator at 10 °C.
Electrophysiology
Membrane currents were recorded from cells under whole cell voltage clamp as previously described [5]. Depolarizing voltage steps were applied and current responses recorded and analyzed using pCLAMP 8.0 and Clampfit 8.0 software (Axon Instruments, Union City, CA) run on a personal computer in conjunction with a Yale designed amplifier and a DigiData 1200 digitizer (Axon Instruments, Union City, CA). Electrode resistances measured in the bath were between 5 and 10 MΩ. Recording pipette internal solution (pH 7.2) consisted of the following (in mM): 105 KCl, 5 MgCl2, 0.05 EGTA, 1 ATP, 10 HEPES, 6 NaOH. The chloride equilibrium potential, ECl, was approximately 0 mV under these conditions. Bath solution containing fluoxetine was administered with a brief puff (about 1-2 s) to cells by positive pressure using a picospritzer from a micropipette with a large diameter (about 50 μm) opening to ensure whole cells were bathed upon ejection. Ejection volume during puff was estimated to be approximately 25 μl and was administered to cells maintained in 1.5 ml static bath solution. Saline solution containing 0.5% DMSO was used as a vehicle-only control. Cells were maintained at 10-12 °C using a cooling stage platform during recordings. All rods used for recordings possessed intact axons and presynaptic terminals.
Peak outward currents induced by a 2 s depolarization from -60 mV to 0 mV were defined as the difference between the maximal current amplitude measured at the end of the voltage step to 0 mV and the baseline current at -60 mV measured prior to the depolarizing voltage step. Similarly, peak ICl(Ca) tail currents following a 2 s depolarizing voltage step from -60 mV to 0 mV were defined as the difference between maximal current amplitude measured during the interval of sustained current for each recording and the baseline current measured at -60 mV prior to onset of the depolarizing voltage step. Peak outward currents induced by a series of 20 ms voltage steps from -60 mV to +40 mV were defined as the difference between the current amplitude at 18 ms following onset of the depolarizing step and the baseline current measured prior to onset of the depolarizing voltage step. These values were then used to calculate the percent inhibition of current effected by fluoxetine as follows:
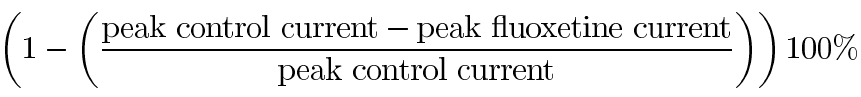
Statistical comparisons of currents employed a Student's t-test analysis to calculate p values. Variation within the data is presented as the standard error of the mean (SEM). Capacitive transients, appearing as sharp inflection points accounted for by single data points, were observed in all electrical traces. These were removed manually from data prior to plotting electrical traces.
Calcium Imaging
Cells were dissociated and plated as previously described and preloaded with fura-2 AM (Molecular Probes, Eugene, OR) at a concentration of 10 μg/ml for 30 min at room temperature. Cells were then rinsed three times with the above described balanced salt solution. Cells were imaged using a thermoelectrically cooled CCD digital camera (Photometrics, Tucson, AZ) in conjunction with Metafluor 6.1 software (Molecular Devices Corporation, Sunnyvale, CA) for fluorescent images and Metamorph 6.1 software for DIC images (Molecular Devices Corporation, Sunnyvale, CA). Cells were excited with dual wavelength light (340 nm or 380 nm) using a Lambda DG-5 ultra high speed wavelength switcher (Sutter Instrument Co., Novato, CA). Emission (510 nm) images were captured using a 50 ms exposure time for each wavelength of light. The rate of data acquisition with the system was approximately 300 msecs per 340/380 nm ratio. Fluorescent emissions were averaged over regions of interest corresponding to the soma (nucleus and perikarya) and the presynaptic terminal. Depolarizing stimuli were provided by brief puffs (1-2 s) of saline solution composed as previously described, but containing 100 or 50 mM KCl (and a corresponding decrease in NaCl concentration), ejected by positive pressure using a picospritzer from a micropipette with a large diameter (about 50 m) opening to ensure whole cells were bathed upon ejection. Cells were maintained at 10-12 °C using a cooling stage platform during recordings. Cells were exposed to bright light using the halogen lamp of the microscope prior to imaging with UV light, making it unlikely that photocurrents of the outer segments affected voltage changes in the somata and terminals during imaging. Representative raw imaging data are presented as fluorescence ratio, which is known to be proportional to intracellular calcium concentration [6], shown over time (s). For purposes of comparison, changes in fura-2 fluorescence are presented as ΔR where ΔR is the maximal 340/380 nm ratio measured following depolarization less the baseline 340/380 nm ratio measured prior to depolarization. Statistical comparisons of changes in fluorescence employed a Student's t-test analysis to calculate p values. Variation within the data is presented as the standard error of the mean (SEM).
Results
Fluoxetine inhibits calcium-activated chloride currents, ICl(Ca)
To test the effects of fluoxetine, we recorded multiple whole cell current responses from individual cells before, during, and after "puffing" fluoxetine hydrochloride onto the same cell using voltage clamp technique. Currents of intact rod photoreceptors possessing axons and presynaptic terminals were recorded in response to a 2 s depolarizing voltage step from -60 mV to 0 mV. The depolarizing voltage step induced a net outward current followed by a tail current indicative of the calcium-activated chloride current, ICl(Ca) [7-9]. A puff of fluoxetine (50 μM pipette concentration), almost totally blocked ICl(Ca) (96%±1.3% SEM; n=9) and outward currents during the depolarizing step were also greatly reduced (75%±2.9% SEM; n=9). When 10 μM fluoxetine (pipette concentration) was used, the amplitude of ICl(Ca) was only slightly reduced (14%±9.3 SEM; n=5), but the duration of the current was substantially attenuated (48%±7.0 SEM; n=5); again, the outward current elicited during the voltage step was also inhibited (23%±6.7 SEM; n=5). No appreciable difference in either the amplitude or the duration of ICl(Ca) was observed when medium containing only DMSO was puffed (n=2) as a vehicle-only control. A representative intact rod and accompanying representative traces and statistical summaries are shown in Figure 1.
Fluoxetine inhibits calcium-dependent outward current, IK(Ca)
The outward and tail currents we observed following depolarization were almost completely blocked by a puff of 50 μM fluoxetine, but inhibited to a much lesser degree by a puff of 10 μM fluoxetine. However, the puffing technique does not allow us to know precisely the actual concentration of fluoxetine exerting the observed effects. Furthermore, a 2 s depolarizing voltage step to 0 mV is a very strong depolarizing stimulus which may not be physiologically relevant. For these reasons, we decided to test the effects of a fixed concentration of fluoxetine in the bath on the outward currents induced by a series of short depolarizing voltage steps. Cells preincubated in 25 μM fluoxetine for 30 min consistently exhibited significantly reduced outward currents compared to cells in control medium during a series of depolarizing voltage steps from -60 mV to +40 mV. Since ICl(Ca) is negligible at 0 mV (ECl approximates 0 mV under our experimental conditions), the bulk of the depolarization-induced current at 0 mV is carried by a calcium-activated K+ current, IK(Ca), along with a voltage-gated, delayed rectifier-type K+ current, IK(V), both of which are functionally localized to the somatic compartment [1]. The reduction (49% reduction; p<0.05) in net depolarization-induced whole cell currents recorded in bath solution containing 25 μM fluoxetine (91.5 pA±10.9 SEM; n=5) compared to currents in control bath solution (187 pA±24.1 SEM; n=7), therefore, reflects an inhibition of IK(Ca) and IK(V). At +40 mV, where currents have both IK(Ca) and ICl(Ca) components, we also observed a significant reduction (35% reduction; p<0.01) in outward currents recorded in bath solution containing 25 μM fluoxetine (274 pA±26 SEM; n=5) compared to currents recorded in control bath solution (783 pA±71 SEM; n=7). The reduced currents observed in cells preincubated in 25 μM fluoxetine are very similar to those recorded in the presence of 150 μM CdCl2 (n=4), which is known to block calcium-dependent currents indirectly by inhibiting calcium entry via voltage-gated calcium channels. Representative traces and a statistical summary from these experiments are shown in Figure 2.
Fluoxetine alters both somatic and terminal intracellular calcium dynamics following membrane depolarization
The above described experiments demonstrated that fluoxetine inhibits two calcium-activated currents, ICl(Ca) and IK(Ca), in salamander rod photoreceptors. The inhibitory effects of fluoxetine on two independent calcium-dependent currents could be accounted for by an underlying alteration of normal intracellular free calcium dynamics following depolarization. To test this hypothesis, we monitored changes in the intracellular free calcium levels as changes in fura-2 fluorescence (340 nm/380 nm ratio) of the somatic and presynaptic terminal compartments of intact isolated rods following depolarization induced by a brief puff of medium containing a high concentration of K+ (100 mM pipette concentration). Administration of high extracellular potassium as a depolarizing stimulus instead of an electrical stimulus administered via patch clamp with an electrode allowed for preservation of the endogenous buffering capacity of the cells. A previous study of calcium dynamics in salamander rods by our lab [10] demonstrated that brief application of 100 mM K+ (pipette concentration) results in a significant elevation of free calcium levels in both the somatic and terminal compartments. For comparison, responses following depolarization in control bath solution or bath solution containing 10 μM fluoxetine were recorded from the same cells.
Following depolarization with 100 mM K+ in control bath solution, a large increase in intracellular calcium levels was observed as a large increase in fura-2 fluorescence (measured as a change in 340/380 nm ratio, ΔR) in both the somatic (ΔR=2.51±0.46 SEM) and presynaptic terminals (ΔR=1.81±0.21 SEM) of rods (n=7). However, preincubation of these same cells for 10 min in bath solution containing 10 μM fluoxetine virtually eliminated the rise in calcium following depolarization in both the somatic (ΔR=0.011±0.005 SEM; 99.6% reduction; p<0.01) and presynaptic terminal compartments (ΔR=0.06±0.016 SEM; 96.7% reduction; p<0.01). A representative cell, accompanying changes in fura-2 fluorescence, and a statistical summary for these experiments are shown Figure 3. The observation of a nearly complete elimination of the response supports the notion that the effective concentration of fluoxetine in our earlier experiments looking at the effects of puff administration of 10 μM fluoxetine on cell currents is in fact lower than the pipette concentration. Interestingly, we were unable to consistently obtain washout responses from cells that had been previously depolarized in the presence of 10 μM fluoxetine. However, we were consistently able to obtain responses from cells with previous exposure to 10 μM fluoxetine but no previous depolarization in its presence.
Since preincubation of cells in 10 μM fluoxetine virtually abolished intracellular calcium responses, we hypothesized that a lower dose might result in a partial inhibition of the responses similar to that observed when puffing on 10 μM fluoxetine. From previous experience, we knew that the required bath concentrations for various drugs were typically 3-4 times lower than those used in puffing experiments to achieve the same effects. We therefore tested 4 μM to try to mimic the partial inhibition of responses we had observed when puffing cells with 10 μM fluoxetine.
This time, we conducted a population study of cells in control bath (n=10) and cells preincubated in 4 μM fluoxetine (n=16) to eliminate concerns about repeated stimulation of the same cells. We also administered medium containing 50 mM K+ (pipette concentration) as a milder depolarizing stimulus in these experiments. A potassium concentration of 50 mM is predicted to depolarize the cell to a value above -18 mV (the Nernst potential for potassium), near the reported peak of the calcium current in photoreceptors, and is therefore likely to produce a maximal or near maximal calcium increase. Following depolarization with 50 mM K+ in control bath solution, a large increase in intracellular calcium levels was observed as a large increase in fura-2 fluorescence in both the somatic (ΔR=0.31±0.04 SEM) and terminal (ΔR=1.22±0.12 SEM) compartments. These increases were smaller than those observed when using 100 mM K+ as the depolarizing stimulus. Preincubation of cells for 10 min in bath solution containing 4 μM fluoxetine significantly reduced, but did not abolish, the rise in calcium following depolarization in both the somatic (ΔR=0.11±0.02 SEM; 64.5% reduction; p<0.001) and presynaptic terminal compartments (ΔR=0.75±0.11 SEM; 38.5% reduction; p<0.02). While the amplitude of the increase in the terminal compartment calcium levels was not as significantly reduced by this lower concentration of fluoxetine, the duration of sustained elevation was more significantly reduced (>50%, p<0.0001). Representative changes in fura-2 fluorescence and a statistical summary for these experiments are shown in Figure 4. These data are consistent with the observation of a small reduction in amplitude of ICl(Ca) tail currents and a larger reduction in the duration of these tail currents when puffing 10 μM fluoxetine. Combined, the fura-2 imaging data demonstrate that inhibition of ICl(Ca) and IK(Ca) is associated with modulation of intracellular calcium dynamics following depolarization and demonstrate that, in addition to any direct effects on the channels underlying ICl(Ca), fluoxetine exerts a modulatory effect on depolarization-induced intracellular calcium dynamics at lower doses and a near total block of depolarization-induced calcium elevations at slightly higher concentrations.
Effects of fluoxetine in the presynaptic terminal are more steeply dose-dependent than those of the somatic compartment
We plotted the mean percent inhibition of terminal ICl(Ca) currents and somatic IK(Ca) currents against the concentrations of fluoxetine used in our experiments and fitted the data with linear functions in order to compare slopes as an estimate of the dose dependency of the effects of fluoxetine on the currents of the two compartments. As illustrated in Figure 5A, the slope of the fitted data for terminal ICl(Ca) is noticeably steeper than that for the somatic IK(Ca) currents. In similar fashion, we plotted the mean changes in depolarization-induced intracellular calcium levels, measured with fura-2 as changes in the 340/380 nm ratios, against the fluoxetine concentrations used in our experiments and fitted the data with linear functiuons in order to compare slopes as an estimate of the dose dependency of the effects of fluoxetine on intracellular calcium changes within the two compartments following depolarization. As illustrated in Figure 5B, the slope of the fitted data for terminal calcium changes is noticeably steeper than that for the somatic calcium changes. Thus, there is an apparent difference in the dose dependency of fluoxetine effects on calcium increases and on calcium-dependent currents of the terminal compartment as compared to those of the somatic compartment of salamander rods.
Discussion
In this study, we set out to determine whether fluoxetine hydrochloride could be used as a specific inhibitor of the calcium-dependent chloride tail current, ICl(Ca) in the terminals of isolated salamander without perturbing other aspects of rod physiology. Our electrophysiological studies demonstrated a near total block of ICl(Ca) in the presence of a 50 μM fluoxetine test puff and a significant reduction in the duration of ICl(Ca) in the presence of a 10 μM fluoxetine test puff, confirming the ability of fluoxetine to inhibit ICl(Ca) in a dose-dependent fashion. However, these same studies also demonstrated a similar degree of inhibition of the depolarization-induced outward current, the bulk of which corresponds to a somatic calcium-dependent potassium current, IK(Ca). Furthermore, the degree of inhibition of depolarization-induced IK(Ca) by preincubation in a bath solution containing 25 μM fluoxetine was similar to that observed when cells were preincubated in CdCl2, a generic blocker of voltage-gated calcium channels. Combined, these electrophysiological data suggested that in addition to any direct effect of fluoxetine on the channels underlying ICl(Ca), fluoxetine may exert an inhibitory effect on ICl(Ca) and IK(Ca) via a reduction in the amplitude and duration of rises in intracellular calcium levels following depolarization.
To test this hypothesis, we monitored the changes in intracellular free calcium dynamics of isolated salamander rods preloaded with fura-2 in control bath solution and bath solution containing fluoxetine following a brief depolarizing puff of high potassium. Changes within both the somatic and presynaptic terminal compartments were monitored as changes in fura-2 fluorescence. Consistent with a dose-dependent modulation of calcium dynamics, we observed a nearly total block of the rise in intracellular calcium levels of both somatic and terminal compartments following depolarization with 100 mM K+ (pipette concentration) when 10 μM fluoxetine was included in the bath solution. While a rise in intracellular calcium levels of both the somatic and terminal compartments was still observed following depolarization with 50 mM K+ (pipette concentration), the level was significantly reduced compared to controls, and a greater and more significant reduction was observed in the duration of the sustained component of the calcium elevation. These data are consistent with an additional effect of fluoxetine on the intracellular calcium dynamics of rods following depolarization, specifically the ability to inhibit the amplitude and duration of elevation of intracellular free calcium within both the somatic and terminal compartments.
One unexpected observation during our imaging experiments was the inability to consistently obtain recovery of normal calcium responses on the same cells previously depolarized in the presence of fluoxetine following washout of the drug. We consistently observed recovered electrical responses on the same cells following washout of fluoxetine administered as a test puff, and we could consistently elicit a normal change in calcium levels following depolarization of rods preexposed to the drug, but not depolarized in its presence. A possible explanation for this apparent paradox is that more fluoxetine makes it into the cells when it is included in the bath solution instead of administered as a brief puff and that fluoxetine exerts a more irreversible effect on channels from inside the cell.
While our data are consistent with the notion that fluoxetine hydrochloride blocks calcium entry via voltage-gated calcium channels in rod photoreceptors, we did not demonstrate this directly by observing the ability of fluoxetine to block isolated calcium currents. As mentioned in the introduction to this study, it is not so straightforward to measure the calcium currents of an intact rod photoreceptor retaining its presynaptic terminal due to the large calcium-dependent chloride current, ICl(Ca), which is functionally localized to the terminal and obscures isolation of the voltage-gated calcium currents. A review of the literature, however, reveals a number of reports demonstrating the blockage of voltage-gated calcium channels by fluoxetine in a variety of other cell types including cardiomyocytes [11], arteriolar smooth muscle cells [12], cochlear neurons [13], and pyramidal neurons from hippocampus and prefrontal cortex and prefrontal cortex [14]. It is therefore highly likely that the changes in intracellular calcium dynamics we observed are also mediated via blockage of the L-type calcium channels expressed in rod photoreceptors. Fluoxetine administration to rats has been reported to induce alterations in the neuroplasticity of the rodent visual system by an unknown mechanism [15]. This observation might be related to the finding of altered plasticity in isolated photoreceptors exposed to calcium channel blockers [16]. In addition to suppression of calcium and calcium-activated channels, fluoxetine has also now been reported to inhibit voltage-gated K+ channels [17-21]. Although we did not directly test the ability of fluoxetine to inhibit the small voltage-gated K+ currents in salamander rods, it is possible that fluoxetine also inhibits these currents.
The observation that ICl(Ca) tail currents and depolarization evoked intracellular calcium changes within the terminal exhibited a steeper dose-dependent relationship to fluoxetine concentration than somatic IK(Ca) outward currents suggests several distinct possibilities. One possibility is that fluoxetine exerts some cooperative inhibitory effect in the terminal but not in the soma. The steeper effect in the terminal could be due to different sites of action or degrees of cooperativity of fluoxetine in the two compartments. Cooperativity in the terminal could indicate another site of inhibitory action of fluoxetine in addition to ICl(Ca), such as release of calcium from intracellular stores. Cooperativity in the terminal could also reflect a tight coupling of ICl(Ca) and capacitive calcium entry into the terminal. As ICl(Ca) will modulate the cell's membrane potential following depolarization and initial calcium influx, it probably plays an important role in regulating intracellular calcium levels by directly modulating the opening and closing of voltage-gated calcium channels and possibly having an indirect impact on calcium-induced calcium release (CICR). It is therefore a distinct possibility that the observed changes in intraterminal calcium dynamics may be, at least in part, the direct consequence of blockage of ICl(Ca). Applying this same logic, IK(Ca) may be less tightly coupled to capacitive calcium entry in the soma, yielding a less steep dependence of inhibition of somatic outward currents and somatic calcium changes on fluoxetine concentration. We recently reported evidence for a concentration of L-type calcium channels with properties distinct from those of the soma in the membrane of the presynaptic terminals of isolated salamander rods [10]. If fluoxetine blocks these channels as effectively as it has been reported to block the voltage-gated calcium channels of other cell types, this could account for the apparent disparity in cooperativity of fluoxetine's effects in the two compartments. Finally, intrinsic differences in the calcium buffering, reuptake, and extrusion mechanisms of the somatic and terminal compartments, which have been highlighted in previous reports [22-24], should not be ignored as a possible explanation of this apparent difference in the cooperativity of fluoxetine effects in the two compartments.
Although it is beyond the original purpose and scope of this study, the widespread use of fluoxetine as an antidepressant entices one to entertain the potential clinical implications of our data. Serum concentrations for fluoxetine have been reported as 272 nM-2.72 μM in patients during the last three weeks of a 20-60 mg per day regiment of fluoxetine [25]. Serum concentrations for norfluoxetine, the active metabolite of fluoxetine, were measured at almost identical levels in this same study. Norfluoxetine has been shown to be equally potent in its ability to block calcium channels in isolated rat cochlear neurons [13]. In the present study, significant effects on calcium-dependent currents and depolarization-induced changes in intracellular free calcium levels were observed when a test puff containing 10 μM fluoxetine was administered (equivalent to about 3.3 μM in the bath based upon previous experience) or when cells were preincubated in a bath solution containing 4 μM fluoxetine. While this concentration is similar to the reported EC50 of 5.4±0.94 μM concentration reported to block peak calcium currents at 0 mV in ventricular cardiomyocytes [11], it is more than the highest concentration measured in the blood serum of patients taking a high (60 mg/day) dose regiment of fluoxetine [25]. On the subject of relevant concentrations of fluoxetine, however, it should be noted that brain concentrations of fluoxetine are reported to be significantly higher than corresponding blood plasma levels, with an apparent ratio of 20:1 [26]. It is therefore possible that retinal concentrations of fluoxetine could also be higher than corresponding blood levels, in which case the effects of fluoxetine on rod photoreceptor currents and visual function could be even greater. If this were true, one would anticipate a reduction in the photoreceptor output resulting in a reduction in the b-wave of the electroretinogram (ERG) and visual deficits. A review of the published literature on side effects of fluoxetine, however, reveals no evidence of visual deficits in patients taking fluoxetine. The present study was conducted using a limited range of concentrations of fluoxetine on amphibian photoreceptors. Nonetheless, it raises the possibility of aberrant rod photoreceptor functioning that may manifest as subtle or even an unappreciated visual deficit in patients taking a therapeutic regimen of fluoxetine. A more intensive study of the effects of various therapeutically relevant concentrations of fluoxetine on the ionic conductance and calcium dynamics of rod photoreceptors from a primate species is required to probe this possibility further.
Acknowledgements
This work was supported by National Institutes of Health research grants R01 NS 35510 and U54 NS 034194. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Grant 1 C06 RR07571 from the National Center for Research Resources, National Institutes of Health. The authors would like to thank Dr. Colin A. Nurse for inspiration and thoughtful discussions related to this study.
References
1. MacLeish PR, Nurse CA. Ion channel compartments in photoreceptors. Invest Ophthalmol Vis Sci 2000; 41:S494.
2. Maertens C, Wei L, Voets T, Droogmans G, Nilius B. Block by
fluoxetine of volume-regulated anion channels. Br J Pharmacol 1999;
126:508-14. ![]()
3. MacLeish PR, Chen X, Steele E. Fluoxetine suppresses the rise in intracellular levels of calcium and blocks IK(Ca) and ICl(Ca) in the salamander rod. Invest Ophthalmol Vis Sci 2001; 42:S119.
4. MacLeish PR, Barnstable CJ, Townes-Anderson E. Use of a monoclonal
antibody as a substrate for mature neurons in vitro. Proc Natl Acad Sci
U S A 1983; 80:7014-8. ![]()
5. Yagi T, Macleish PR. Ionic conductances of monkey solitary cone
inner segments. J Neurophysiol 1994; 71:656-65. ![]()
6. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+
indicators with greatly improved fluorescence properties. J Biol Chem
1985; 260:3440-50. ![]()
7. Bader CR, Bertrand D, Schwartz EA. Voltage-activated and
calcium-activated currents studied in solitary rod inner segments from
the salamander retina. J Physiol 1982; 331:253-84. ![]()
8. Maricq AV, Korenbrot JI. Calcium and calcium-dependent chloride
currents generate action potentials in solitary cone photoreceptors.
Neuron 1988; 1:503-15. ![]()
9. Barnes S, Hille B. Ionic channels of the inner segment of tiger
salamander cone photoreceptors. J Gen Physiol 1989; 94:719-43.
![]()
10. Steele EC Jr, Chen X, Iuvone PM, MacLeish PR. Imaging of Ca2+
dynamics within the presynaptic terminals of salamander rod
photoreceptors. J Neurophysiol 2005; 94:4544-53. ![]()
11. Pacher P, Magyar J, Szigligeti P, Banyasz T, Pankucsi C, Korom Z,
Ungvari Z, Kecskemeti V, Nanasi PP. Electrophysiological effects of
fluoxetine in mammalian cardiac tissues. Naunyn Schmiedebergs Arch
Pharmacol 2000; 361:67-73. ![]()
12. Ungvari Z, Pacher P, Koller A. Serotonin reuptake inhibitor
fluoxetine decreases arteriolar myogenic tone by reducing smooth muscle
[Ca2+]i. J Cardiovasc Pharmacol 2000; 35:849-54. ![]()
13. Kecskemeti V, Rusznak Z, Riba P, Pal B, Wagner R, Harasztosi C,
Nanasi PP, Szucs G. Norfluoxetine and fluoxetine have similar
anticonvulsant and Ca2+ channel blocking potencies. Brain Res Bull 2005;
67:126-32. ![]()
14. Deak F, Lasztoczi B, Pacher P, Petheo GL, Kecskemeti V, Spat A.
Inhibition of voltage-gated calcium channels by fluoxetine in rat
hippocampal pyramidal cells. Neuropharmacology 2000; 39:1029-36.
![]()
15. Bastos EF, Marcelino JL, Amaral AR, Serfaty CA.
Fluoxetine-induced plasticity in the rodent visual system. Brain Res
1999; 824:28-35. ![]()
16. Nachman-Clewner M, St Jules R, Townes-Anderson E. L-type calcium
channels in the photoreceptor ribbon synapse: localization and role in
plasticity. J Comp Neurol 1999; 415:1-16. ![]()
17. Choi JS, Hahn SJ, Rhie DJ, Yoon SH, Jo YH, Kim MS. Mechanism of
fluoxetine block of cloned voltage-activated potassium channel Kv1.3. J
Pharmacol Exp Ther 1999; 291:1-6. ![]()
18. Bian JT, Yeh JZ, Aistrup GL, Narahashi T, Moore EJ. Inhibition of
K+ currents of outer hair cells in guinea pig cochlea by fluoxetine. Eur
J Pharmacol 2002; 453:159-66. ![]()
19. Choi BH, Choi JS, Ahn HS, Kim MJ, Rhie DJ, Yoon SH, Min DS, Jo
YH, Kim MS, Hahn SJ. Fluoxetine blocks cloned neuronal A-type K+
channels Kv1.4. Neuroreport 2003; 14:2451-5. ![]()
20. Terstappen GC, Pellacani A, Aldegheri L, Graziani F, Carignani C,
Pula G, Virginio C. The antidepressant fluoxetine blocks the human small
conductance calcium-activated potassium channels SK1, SK2 and SK3.
Neurosci Lett 2003; 346:85-8. ![]()
21. Yeung SY, Millar JA, Mathie A. Inhibition of neuronal KV
potassium currents by the antidepressant drug, fluoxetine. Br J
Pharmacol 1999; 128:1609-15. ![]()
22. Krizaj D, Copenhagen DR. Compartmentalization of calcium
extrusion mechanisms in the outer and inner segments of photoreceptors.
Neuron 1998; 21:249-56. ![]()
23. Krizaj D, Lai FA, Copenhagen DR. Ryanodine stores and calcium
regulation in the inner segments of salamander rods and cones. J Physiol
2003; 547:761-74. ![]()
24. Morgans CW, El Far O, Berntson A, Wassle H, Taylor WR. Calcium
extrusion from mammalian photoreceptor terminals. J Neurosci 1998;
18:2467-74. ![]()
25. Orsulak PJ, Kenney JT, Debus JR, Crowley G, Wittman PD.
Determination of the antidepressant fluoxetine and its metabolite
norfluoxetine in serum by reversed-phase HPLC with ultraviolet
detection. Clin Chem 1988; 34:1875-8. ![]()
26. Karson CN, Newton JE, Livingston R, Jolly JB, Cooper TB, Sprigg
J, Komoroski RA. Human brain fluoxetine concentrations. J
Neuropsychiatry Clin Neurosci 1993; 5:322-9. ![]()