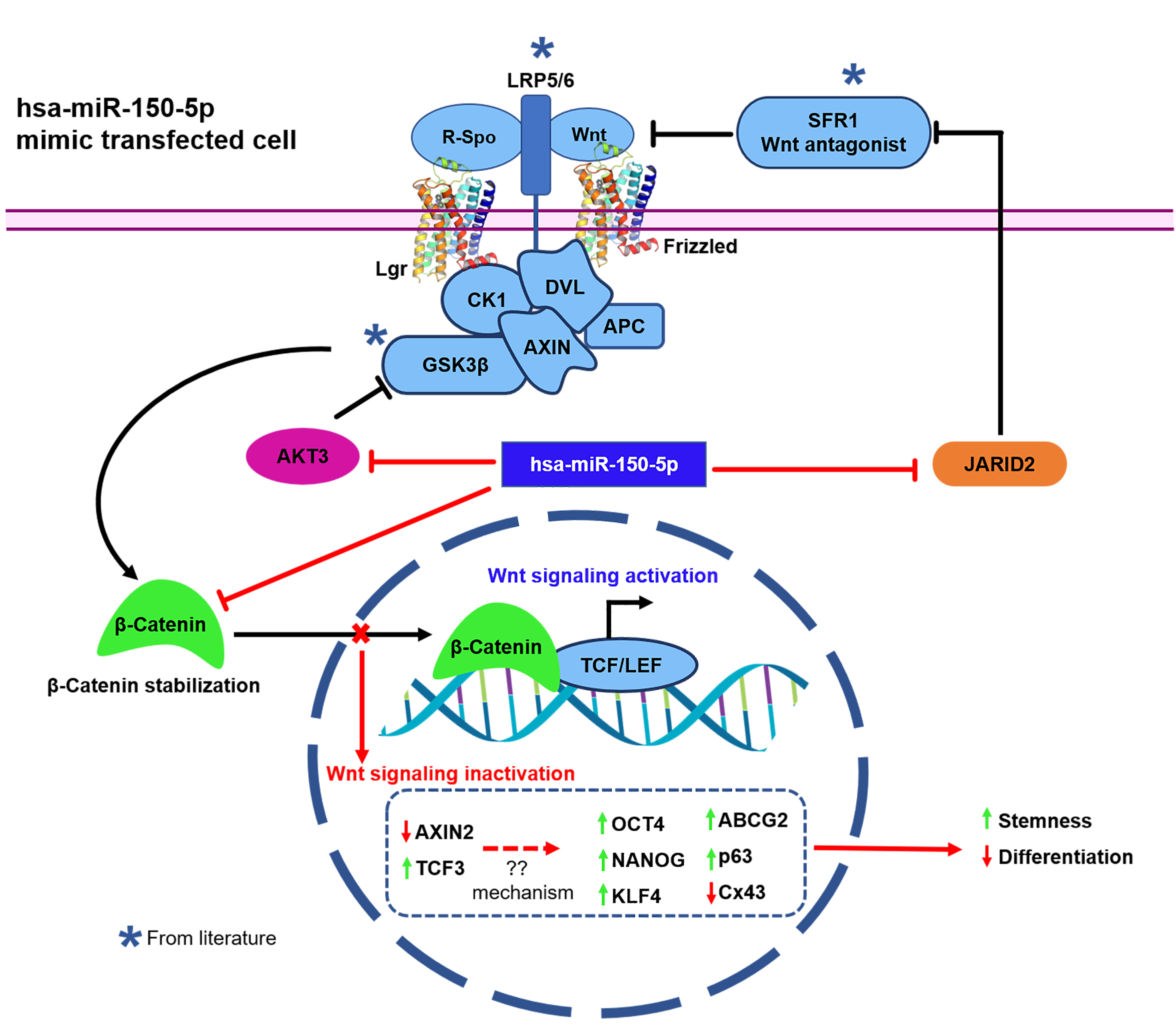
Figure 7. Mechanism of the regulation of Wnt-β-catenin signaling by hsa-miR-150-5p. In the presence of Wnt ligands and Wnt agonists
such as R-Spondin (R-Spo), cytoplasmic disheveled (Dvl) is recruited to the membrane receptors, resulting in subsequent phosphorylation
and activation of low-density lipoprotein receptor-related protein 5/6 (LRP5/6). These events lead to the titration of the
active destruction complex composed of glycogen synthase kinase 3 (GSK3β), casein kinase I (CK1), adenomatosis polyposis coli
(APC), and Axin. As a consequence, β-catenin is stabilized and accumulated in the cytoplasm, resulting in the nuclear translocalization
of β-catenin, where it activates Wnt signaling through its interaction with the TCF/LEF (T-cell factor/lymphoid-enhancing
factor) nuclear complex [
57,
58]. Hsa-miR-150-5p prevents the activation of Wnt signaling (red lines) by reducing the expression levels of its targets: (i)
β-catenin-Wnt signaling activator (
Figure 1B,
Figure 2,
Figure 3,
Figure 5, and
Figure 6), (ii) the AKT3 enhancer of β-catenin stabilization through inhibition of GSK3β (
Figure 1B,
Figure 2,
Figure 3, and
Figure 5), and (iii) the JARID2 activator of Wnt signaling through the repression of Wnt antagonist-secreted frizzled-related protein
1 (SFRP1;
Figure 1B,
Figure 2,
Figure 3, and
Figure 5). This results in a decreased expression level of the Wnt target gene
AXIN2 and an increased expression level of
TCF3, a Wnt signaling transcriptional repressor.
 Figure 7 of
Kalaimani, Mol Vis 2022; 28:178-191.
Figure 7 of
Kalaimani, Mol Vis 2022; 28:178-191.  Figure 7 of
Kalaimani, Mol Vis 2022; 28:178-191.
Figure 7 of
Kalaimani, Mol Vis 2022; 28:178-191.