Received 14 February 2007 | Accepted 12 September 2007 | Published 18 September 2007
Reprint
Received 14 February 2007 | Accepted 12 September 2007 | Published 18 September 2007 |
Download Reprint |
NADH photo-oxidation is enhanced by a partially purified λ-crystallin fraction from rabbit lens
Masayasu Bando,1
Mikako Oka,2
Kenji Kawai,1
Hajime Obazawa,3
Makoto Takehana2
1Department of Ophthalmology, Tokai University School of Medicine, Isehara, Japan; 2Department of Molecular Function and Physiology, Kyoritsu University of Pharmacy, Tokyo, Japan; 3Eye Research Institute of Cataract Foundation, Tokyo, Japan
Correspondence to: Makoto Takehana, Ph.D., Department of Molecular Function and Physiology, Kyoritsu University of Pharmacy, 1-5-30 Shibakoen, Minato-ku, Tokyo 105-8512, Japan; Phone: +81-3-5400-2663; FAX: +81-3-5400-2693; email: takehana-mk@kyoritsu-ph.ac.jp
Abstract
Purpose: In the rabbit lens, high levels of reduced nicotinamide adenine dinucleotide (NADH) can function as a near-ultraviolet light (near-UV) filter, an effect apparently achieved by specific nucleotide binding to λ-crystallin. The present investigation asks whether λ-crystallin enhances NADH photo-oxidation by superoxide radicals produced via a photosensitization reaction of near-UV with NADH.
Methods: λ-Crystallin was partially purified from rabbit lens soluble fraction by a two-step gel filtration and affinity column chromatography procedure. NADH solutions with or without partially purified λ-crystallin were subjected to near-UV irradiation or exposed to superoxide generated enzymatically by the xanthine/xanthine oxidase system. NADH oxidation was determined by assaying the decrease of absorbance at 340 nm.
Results: When irradiated with near-UV, free NADH was oxidized very little in the absence of λ-crystallin. In contrast, NADH photo-oxidation was rapidly initiated in the presence of partially purified λ-crystallin. This λ-crystallin-enhanced NADH photo-oxidation was totally inhibited by adding superoxide dismutase. We also found that λ-crystallin largely increased NADH oxidation by a superoxide that is generated enzymatically. These results indicate that NADH bound to λ-crystallin rapidly reacts with superoxides. The reactivity of bound NADH with superoxide was almost equivalent to that of ascorbic acid. However, λ-crystallin-enhanced NADH oxidation exceeded the superoxide levels generated by NADH photosensitization and xanthine/xanthine oxidase.
Conclusions: We conclude that NADH binding to λ-crystallin enhances NADH photo-oxidation through a radical chain reaction mechanism that is initiated by superoxides generated by NADH photosensitization and propagated by another superoxide from the molecule oxygen. High concentrations of NADH bound to λ-crystallin may be beneficial to the rabbit lens in scavenging the low amounts of superoxide produced by near-UV absorption, since oxygen tension in the lens is very low. We also discuss the function of near-UV-filtering and the anti-photo-oxidation systems in other vertebrate lenses.
Introduction
A function of the eye lens is to absorb near-ultraviolet light (near-UV) transmitted through the cornea and aqueous humor in the wavelength range of about 300-400 nm [1-3], thereby preventing UV-induced photo-damage to the retina [4,5] and reducing chromatic aberration [3]. It has been reported that the majority of environmental UV radiation reaching the lens is UV-A (320-400 nm) and that only approximately 3% is UV-B (290-320 nm) [6]. Several investigators [7-9] have shown that long-term exposure of mice and guinea pigs to near-UV can damage the lens, probably due to photo-oxidation. It is assumed that normally the lens is protected from photo-oxidation by anti-oxidative systems utilizing the ascorbate [10] and glutathione [11] redox cycles. However, the anti-photo-oxidation systems in the lens are not yet fully understood.
Zigler and Rao [12] reported that the lenses of vertebrate species such as bullfrogs, guinea pigs, ducks, and rabbits contain high concentrations (0.4-1.5 mM) of the reduced pyridine nucleotides reduced nicotinamide adenine dinucleotide phosphate (NADPH) or reduced nicotinamide adenine dinucleotide (NADH), which may function as near-UV filters. High nucleotide levels in the lens are likely due to specific nucleotides binding to taxon-specific enzyme-crystallins related to NAD(P)H/NAD(P)+-dependent oxidoreductases (i.e., ρ-crystallin/aldo-keto reductase in bullfrogs, ζ-crystallin/alcohol dehydrogenase in guinea pigs, ε-crystallin/lactate dehydrogenase B in ducks, and λ-crystallin/hydroxyacyl-CoA dehydrogenase in rabbits) [12-14]. Rao and Zigler [14] have further shown that bullfrogs, guinea pigs, and rabbits lenses exhibiting high levels of NAD(P)H are less susceptible to photo-oxidative damage compared with lenses from the rat, Xenopus, and a mutant strain of guinea pig with lower levels of NAD(P)H.
Cunningham et al. [15,16] have reported that when irradiated with near-UV, NAD(P)H gives rise to low but significant levels of superoxide radicals via a type 2 photosensitization. Matsukura et al. [17] reported that soluble ascorbate free radical (AFR) reductase activity tends to be high in animal lenses and functions as a near-UV filter, enhancing the anti-photo-oxidative capacity of ascorbate. Animal lenses with a near-UV filter may be able to efficiently and rapidly scavenge for superoxides that are photosensitized by NAD(P)H using the ascorbate-AFR reductase system. Thus, it is of interest to investigate whether NAD(P)H binding to enzyme-crystallins decreases and/or modulates the efficiency of superoxide generation by NAD(P)H photosensitization.
λ-Crystallin is a structural lens protein found only in rabbit and hare lenses as reported by Mulders et al. [18]. This group have shown that λ-crystallin shows 30% homology to 3-hydroxyacyl-CoA dehydrogenase and contains a putative NADH-binding site. Recently, Ishikura et al. [19] have reported that λ-crystallin is identical to the active enzyme, NAD+-linked L-gulonate 3-dehydrogenase, which functions in the uronate cycle. Suzuki et al. [20] have also shown that this enzyme-crystallin is related to NADH-dependent dehydroascorbate reductase, based on partial enzyme purification and western blot analysis. These investigations indicate that λ-crystallin is a multifunctional protein that is capable of binding NADH/NAD+. More recently, we [21] have found that most of the NADH binding to partially purified λ-crystallin is tight and nondialyzable and estimated that a dissociation constant of the tight NADH binding is less than 5 nM.
In the present investigation, we demonstrate that λ-crystallin partially purified from a rabbit lens soluble fraction enhances NADH photo-oxidation by superoxide radicals generated by NADH photosensitization. λ-Crystallin also increased NADH oxidation by superoxides generated enzymatically using the xanthine/xanthine oxidase system [22]. NADH bound to enzyme-crystallins rapidly reacted with superoxide through a radical chain reaction mechanism. Free NADH was oxidized very little in the absence of λ-crystallin.
Methods
Preparation of a lens soluble fraction
Ten rabbit lenses were obtained from freshly enucleated eyes of Japanese albino rabbits (6-20 months old) sacrificed with overdoses of an anesthetic containing an equal mixture of 5% ketamine-HCl and 2% xylazine-HCl. Lenses were kept frozen at -80 °C until use. All animal procedures were in accordance with the ARVO resolution on animals and ophthalmic research. Lenses were homogenized 5-10 times (w/v) 0.1 M KCl, 10 mM K-phosphate, pH 7.2 in a glass homogenizer on ice and the soluble fraction was obtained by centrifugation at 15,000x g for 1 h at 4 °C.
Preparation of λ-crystallin by column chromatography
λ-Crystallin was partially purified from the lens soluble fraction by a two-step isolation procedure at 4 °C comprised of gel filtration (Sephadex G-75 superfine or Sephacryl S-200HR; Amersham Biosciences, Piscataway, NJ) and Affi-Gel Blue (Bio-Rad Laboratories, Hercules, CA) affinity-column chromatography as reported previously [21]. A solution of the enzyme-crystallin obtained was dialyzed twice against 50 volumes of 10 mM K-phosphate, 0.1 mM EDTA, pH 7.2 and used for experiments. As described in detail in our previous paper [21], the purity of the 33 kDa protein (the main subunit of λ-crystallin) in the partially purified enzyme-crystallin fraction was about 90% as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Most of the impurities (about 10%) were partial degradation products of the enzyme-crystallin. Protein concentration was assayed by the bicinchoninic acid method [23] using bovine serum albumin as the standard.
Reduced nicotinamide adenine dinucleotide (NADH) photo-oxidation by near-ultraviolet irradiation
NADH (10-50 μM; Wako Pure Chemical Industries, Osaka, Japan) or NADPH (50 μM; Oriental Yeast Company, Tokyo, Japan) was dissolved in 2 ml of 50 mM K-phosphate, 0.1 mM EDTA, pH 7.2 in the presence or absence of partially purified λ-crystallin (0-70 μg protein/ml). The sample was irradiated cumulatively for 2 h at room temperature with near-UV. Control samples were incubated in the dark. In some experiments, 20 μg/ml superoxide dismutase (SOD; lyophilized powder from bovine erythrocytes, about 5,000 U/mg; Boehringer Mannheim GmbH, Mannheim, Germany) was added to the sample before near-UV irradiation. Near-UV, absorbed predominantly by NAD(P)H, was provided by a Toshiba FL 20S-BLB lamp (300-430 nm, maximum at 360 nm; Toshiba, Tokyo, Japan). The lamp was set at a position 8 cm above the surface of the sample solution, and light intensity measured using a J-221 Blak-Ray Ultraviolet Intensity Meter (UVP, Upland, CA) was 590-630 μW/cm2. NAD(P)H oxidation was determined by measuring the decrease in absorbance at 340 nm at cumulative irradiation times of 30, 60, and 120 min. We employed 6,200 M-1cm-1 as the molar extinction coefficient of NAD(P)H at 340 nm [10].
Detection of superoxide generated by reduced nicotinamide adenine dinucleotide (NADH) photosensitization
Superoxide radical generated by NADH photosensitization was detected by measuring spectrophotometrically superoxide-mediated reduction of cytochrome C at 550 nm, using a modified method of Cunningham et al. [15]. Cytochrome C (0.12-0.48 mg/ml, from horse heart, biochemical grade, greater than or equal to 95% purity; Wako Pure Chemical Industries, Osaka, Japan) was added to 50 μM-NADH solutions in the presence or absence of 20 μg/ml SOD. Mixtures were irradiated by near-UV, and the reduction of cytochrome C was measured by monitoring an increase in absorbance at 550 nm. Control samples were incubated in the dark or without NADH to correct for absorbance changes at 550 nm in the dark reaction and in the near-UV absorption of cytochrome C. The amount of superoxide radical generated by NADH photosensitization was calculated from the SOD-inhibited fraction of the cytochrome C photoreduction reaction. The differential molar extinction coefficient (reduced-oxidized) for reduction of cytochrome C at 550 nm was Δε=21,000 M-1cm-1 [22].
Reduced nicotinamide adenine dinucleotide (NADH) oxidation by superoxide generated in a xanthine/xanthine oxidase system
Oxidation of NADH by superoxide radicals in the absence and presence of λ-crystallin was also evaluated using the xanthine/xanthine oxidase system, a well-known enzymatic superoxide generator [22]. Rates of NADH oxidation were measured at 27 °C by recording the absorbance decrease at 340 nm for two min. The reaction system in 50 mM K-phosphate, 0.1 mM EDTA, pH 7.2 at a final volume of 1 ml contained 41-163 μg protein/ml of partially purified λ-crystallin, 50 μM NADH, 20 μM xanthine (Sigma, St. Louis, MO), and 5.5 μg/ml xanthine oxidase (lyophilized powder from buttermilk, 0.25 U/mg; Oriental Yeast Company, Tokyo, Japan). Reactions were initiated by addition of xanthine oxidase. Control experiments omitted xanthine oxidase or λ-crystallin. In some experiments, 0.025-0.21 μg/ml SOD or 2.5-20 μM ascorbic acid was added prior to initiating the reaction. Using the method of cytochrome C reduction [22], we determined that oxidation of 20 μM xanthine by 5.5 μg/ml xanthine oxidase generated superoxide at an initial rate of 0.7 μM/min.
Results
Reduced nicotinamide adenine dinucleotide (NADH) photo-oxidation enhanced by λ-crystallin
Very little of the 50 μM free NADH was oxidized in the absence of λ-crystallin over 2 h of near-UV irradiation (Figure 1A, blue line). In contrast, NADH photo-oxidation was rapidly initiated in the presence of partially purified λ-crystallins (about 90% purity; Figure 1A, red line), which was prepared from rabbit lenses by a two-step gel filtration and affinity column chromatography procedure [21]. This enhanced photo-oxidation was specific for NADH, since it did not occur when NADPH was used instead of NADH (Figure 1B). Weaker NADH photo-oxidation was also observed in crude samples of λ-crystallins separated by gel filtration of a rabbit lens soluble fraction although there was considerable variation in the experimental data (data not shown). These findings indicate that λ-crystallins, which selectively binds to NADH, enhances NADH photo-oxidation. As shown in Figure 2, the rates of NADH photo-oxidation increased proportional to the concentrations of both partially purified λ-crystallin (Figure 2A) and NADH (Figure 2B). λ-Crystallin-enhanced NADH photo-oxidation was totally inhibited by the addition of 20 μg/ml SOD (Figure 3), implying that superoxide radicals were responsible for this NADH photo-oxidation.
Levels of detected superoxide radical generated by photosensitization of 50 μM NADH almost reached saturation in the range of 0.12-0.48 mg/ml cytochrome C, regardless of significantly interfering near-UV absorption by cytochrome C (Figure 4). Saturated levels of superoxide detected were about 2 μM/2 h, and this value was consistent with the photo-oxidation rate (Figure 2A) of 50 μM free NADH without λ-crystallin. However, NADH photo-oxidation rates in the presence of 17.5-70 μg protein/ml of partially purified λ-crystallin, which was about 6-18 μM/2 h (Figure 2A), were three to nine times higher than the measured levels of superoxide photosensitized by NADH. λ-Crystallin-enhanced NADH photo-oxidation apparently exceeds the level of superoxides generated by NADH photosensitization.
λ-Crystallin-enhanced reduced nicotinamide adenine dinucleotide (NADH) oxidation by superoxides generated enzymatically
λ-Crystallin also enhanced NADH oxidation by superoxide radicals that were generated enzymatically using the xanthine/xanthine oxidase system (Figure 5). NADH oxidation rates increased with increasing amounts of partially purified λ-crystallin (Figure 5) and NADH (data not shown). The increased oxidation rates at 50 μM NADH, about 1-5 μM/min in these experiments, were two to seven times higher than the rate (0.7 μM/min) of superoxide generation by xanthine/xanthine oxidase without enzyme-crystallin. Enhanced oxidation of pyridine nucleotide was not observed when NADPH was used instead of NADH (data not shown). As shown in Figure 6A,B, λ-crystallin-enhanced NADH oxidation in the xanthine/xanthine oxidase system was inhibited by ascorbic acid, a low-molecular-weight compound with effective superoxide-scavenging activity [24], or SOD. We estimated that NADH oxidation at 82 μg protein/ml (2.5 μM) of partially purified λ-crystallin was inhibited about 50% by addition of 4 μM ascorbic acid or 0.02 μg/ml (0.6 nM) SOD.
Discussion
The present investigation clearly shows that λ-crystallins partially purified from a rabbit lens soluble fraction enhances NADH oxidation by the superoxide radicals generated by either NADH photosensitization or xanthine/xanthine oxidase. However, enhanced NADH oxidation exceeds the superoxide level generated without the enzyme-crystallin. Chan and Bielski [25-27] reported that in the presence of some dehydrogenases (DH), such as lactate dehydrogenase (pig heart) or glyceraldehyde-3-phosphate dehydrogenase (rabbit muscle), a chain oxidation of NADH is initiated by superoxide radical (O2-/HO2) generated by xanthine/xanthine oxidase or high-energy ionizing radiation and is propagated by oxygen as shown in Reactions 1 to 4 (below). Dehydrogenase (DH)-bound NADH reacts with superoxide (Reaction 2) and the resulting nucleotide radical then reacts with molecular oxygen to generate another superoxide (Reaction 3). Depending on the amount of DH present, oxidation of bound NADH may be repeated many times.
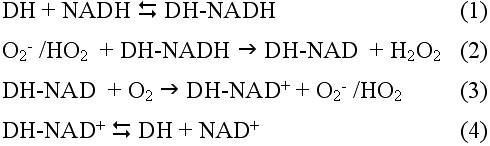
This mechanism is also applicable to λ-crystallin-enhanced NADH oxidation.
The rabbit lens contains small amounts of SOD [28] but high concentrations of ascorbic acid [29] and glutathione [11], which function as superoxide scavengers. Ascorbic acid is apparently more effective at scavenging superoxide than glutathione [30]. It is reported that the rate constant of the reaction with superoxide at neutral pH is 2x109 M-1s-1 for SOD [22] and 2.7x105 M-1s-1 for ascorbic acid [24]. The results in Figure 6A indicate that the superoxide-scavenging activity of λ-crystallins with bound NADH is almost equivalent to that of ascorbic acid. The rate constant for the reaction of NADH bound to λ-crystallin with superoxide is calculated to be 3.5x105 M-1s-1, based on data shown in Figure 6A,B and the method of Sawada and Yamazaki [31]. This calculated rate constant is comparable to the kinetic constants of superoxide-induced NADH oxidation enhanced by lactate and glyceraldehyde-3-phosphate dehydrogenases as estimated by Chan and Bielski [26,27]. However, Petrat et al. [32] reported that lactate dehydrogenase-bound NADH can be characterized as a pro-oxidant since the chain oxidation of bound NADH produces superoxide from oxygen and continuously generates hydrogen peroxide, an additional oxidant. Anti- or pro-oxidative properties of NADH bound to dehydrogenases probably depend on concentrations of oxygen in its environment. Rao and Zigler [14] have reported that the rabbit lens with its high level of NADH is less susceptible to damage by near-UV irradiation. Oxygen tension in the lens, which contains exceptionally high concentrations of reducing compounds such as glutathione and ascorbic acid, is very low, especially in vivo (approximately 2 mmHg or roughly 2% of arterial oxygen tension) [33]. Superoxide-scavenging activity of λ-crystallins with bound NADH may contribute to the high resistance of the rabbit lens to photo-damage. When superoxide radicals are generated in the lens by NADH photosensitization following near-UV absorption, it can be rapidly scavenged locally by high concentrations of NADH bound to the enzyme-crystallin. Radicals propagated from low amounts of molecular oxygen can also be scavenged: SOD, ascorbic acid, and glutathione cooperate to scavenge superoxide. In addition, the glutathione-redox cycle [11] and catalase [28] efficiently detoxify hydrogen peroxide which is produced by superoxide scavenging. Consequently, the rabbit lens is thought to be sufficiently protected from near-UV photo-damage.
Besides λ-crystallins, there exist many taxon-specific enzyme-crystallins binding NAD(P)H/NAD(P)+, which are likely responsible for UV absorption in the vertebrate lens [12,34]. Examples include ε-crystallin in avian and crocodile lenses, ζ-crystallin in guinea-pig and camel lenses, η-crystallin in elephant-shrew lenses, π-crystallin in gecko lenses, and ρ-crystallin in bullfrog lenses. ε-, π-, and η-Crystallins are identical to lactate dehydrogenase B [35], glyceraldehyde-3-phosphate dehydrogenase [36], and retinal dehydrogenase (a class 1 aldehyde dehydrogenase) [37], respectively. ζ-Crystallin is a novel NADPH: quinone oxidoreductase [38]. ρ-Crystallin belongs to an aldo-keto reductase superfamily that includes aldehyde and aldose reductases [34,39], which have recently been reported to catalyze reduction of aldehydes generated during peroxidation of unsaturated fatty acids and phospholipids [40,41]. Therefore, like λ-crystallin, these enzyme-crystallins may have anti-oxidative activities such as superoxide scavenging that protect the lens from near-UV photo-damage.
High levels of enzyme-crystallins related to NAD(P)H/NAD(P)+-dependent oxidoreductases have not been found in the human lens, but very high activities of lactate dehydrogenase [42], glyceraldehyde-3-phosphate dehydrogenase [42], and aldehyde dehydrogenase [43-45] have been detected in the human lens epithelium. NADH bound to these dehydrogenases may selectively absorb significant amounts of near-UV, and these enzymes may play anti-oxidative roles. It is recently reported that an isozyme (ALDH1A1) of aldehyde dehydrogenase in rat lens and human lens epithelial cells detoxifies 4-hydroxynonenal, a highly toxic lipid peroxidation product [46]. Furthermore, the human lens is known to synthesize other near-UV-filtering compounds such as 3-hydroxykynurenine (3-HK)-related glucosides (yellow pigments) from tryptophan and its metabolites [3,47,48]. The yellow-colored glucosides appear to be relatively inefficient photosensitizers [49,50]. Although NAD(P)H is colorless, 3-HK-related glucosides absorb strongly near-UV and weakly violet-blue light at approximately 400-450 nm [3,6]. 3-HK-related glucosides in the human lens may protect the eye not only from near-UV but also from violet-blue light, which is more harmful than light of longer wavelengths [4,5].
References
1. Kinsey VE. Spectral transmission of the eye to ultraviolet radiations. Arch Ophthalmol 1948; 39:508-13.
2. Boettner EA, Wolter JR. Transmission of the ocular media. Invest Ophthalmol 1962; 1:776-83.
3. van Heyningen R. The glucoside of 3-hydroxykynurenine and other fluorescent compounds in the human lens. In: The human lens-- in relation to cataract. New York: Elsevier; 1973. p. 151-71.
4. Ham WT Jr, Mueller HA, Sliney DH. Retinal sensitivity to damage
from short wavelength light. Nature 1976; 260:153-5. ![]()
5. Ham WT Jr, Mueller HA, Ruffolo JJ Jr, Guerry D 3rd, Guerry RK.
Action spectrum for retinal injury from near-ultraviolet radiation in
the aphakic monkey. Am J Ophthalmol 1982; 93:299-306. ![]()
6. Dillon J, Zheng L, Merriam JC, Gaillard ER. The optical properties
of the anterior segment of the eye: implications for cortical cataract.
Exp Eye Res 1999; 68:785-95. ![]()
7. Zigman S, Vaughan T. Near-ultraviolet light effects on the lenses
and retinas of mice. Invest Ophthalmol 1974; 13:462-5. ![]()
8. East EJ, Chang RC, Yu NT, Kuck JF Jr. Raman spectroscopic
measurement of total sulfhydryl in intact lens as affected by aging and
ultraviolet irradiation. Deuterium exchange as a probe for accessible
sulfhydryl in living tissue. J Biol Chem 1978; 253:1436-41.
![]()
9. Barron BC, Yu NT, Kuck JF Jr. Raman spectroscopic evaluation of
aging and long-wave UV exposure in the guinea pig lens: a possible model
for human aging. Exp Eye Res 1988; 46:249-58. ![]()
10. Bando M, Obazawa H. Ascorbate free radical reductase and
ascorbate redox cycle in the human lens. Jpn J Ophthalmol 1988;
32:176-86. ![]()
11. Reddy VN, Giblin FJ. Metabolism and function of glutathione in
the lens. Ciba Found Symp 1984; 106:65-87. ![]()
12. Zigler JS Jr, Rao PV. Enzyme/crystallins and extremely high
pyridine nucleotide levels in the eye lens. FASEB J 1991; 5:223-5.
![]()
13. Rao PV, Zigler JS Jr. Extremely high levels of NADPH in guinea
pig lens: correlation with zeta-crystallin concentration. Biochem
Biophys Res Commun 1990; 167:1221-8. ![]()
14. Rao CM, Zigler JS Jr. Levels of reduced pyridine nucleotides and
lens photodamage. Photochem Photobiol 1992; 56:523-8. ![]()
15. Cunningham ML, Johnson JS, Giovanazzi SM, Peak MJ.
Photosensitized production of superoxide anion by monochromatic (290-405
nm) ultraviolet irradiation of NADH and NADPH coenzymes. Photochem
Photobiol 1985; 42:125-8. ![]()
16. Cunningham ML, Krinsky NI, Giovanazzi SM, Peak MJ. Superoxide
anion is generated from cellular metabolites by solar radiation and its
components. J Free Radic Biol Med 1985; 1:381-5. ![]()
17. Matsukura S, Bando M, Obazawa H, Oka M, Takehana M. Ascorbate
free radical reductase activity in vertebrate lenses of certain species.
Jpn J Ophthalmol 2001; 45:233-9. ![]()
18. Mulders JW, Hendriks W, Blankesteijn WM, Bloemendal H, de Jong
WW. Lambda-crystallin, a major rabbit lens protein, is related to
hydroxyacyl-coenzyme A dehydrogenases. J Biol Chem 1988; 263:15462-6.
![]()
19. Ishikura S, Usami N, Araki M, Hara A. Structural and functional
characterization of rabbit and human L-gulonate 3-dehydrogenase. J
Biochem (Tokyo) 2005; 137:303-14. ![]()
20. Suzuki T, Bando M, Oka M, Tsukamoto H, Akatsuka I, Kawai K,
Obazawa H, Kobayashi S, Takehana M. lambda-crystallin related to
dehydroascorbate reductase in the rabbit lens. Jpn J Ophthalmol 2003;
47:437-43. ![]()
21. Bando M, Oka M, Kawai K, Obazawa H, Kobayashi S, Takehana M. NADH
binding properties of rabbit lens lambda-crystallin. Mol Vis 2006;
12:692-7 <http://www.molvis.org/molvis/v12/a77/>. ![]()
22. Crapo JD, McCord JM, Fridovich I. Preparation and assay of
superoxide dismutases. Methods Enzymol 1978; 53:382-93. ![]()
23. Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH,
Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of
protein using bicinchoninic acid. Anal Biochem 1985; 150:76-85. Erratum
in: Anal Biochem 1987; 163:279. ![]()
24. Nishikimi M. Oxidation of ascorbic acid with superoxide anion
generated by the xanthine-xanthine oxidase system. Biochem Biophys Res
Commun 1975; 63:463-8. ![]()
25. Chan PC, Bielski BH. Enzyme-catalyzed free radical reactions with
nicotinamide adenine nucleotides. II. Lactate dehydrogenase-catalyzed
oxidation of reduced nicotinamide adenine dinucleotide by superoxide
radicals generated by xanthine oxidase. J Biol Chem 1974; 249:1317-9.
![]()
26. Bielski BH, Chan PC. Re-evaluation of the kinetics of lactate
dehydrogenase-catalyzed chain oxidation of nicotinamide adenine
dinucleotide by superoxide radicals in the presence of
ethylenediaminetetraacetate. J Biol Chem 1976; 251:3841-4.
![]()
27. Chan PC, Bielski BH. Glyceraldehyde-3-phosphate
dehydrogenase-catalyzed chain oxidation of reduced nicotinamide adenine
dinucleotide by perhydroxyl radicals. J Biol Chem 1980; 255:874-6.
![]()
28. Bhuyan KC, Bhuyan DK. Superoxide dismutase of the eye: relative
functions of superoxide dismutase and catalase in protecting the ocular
lens from oxidative damage. Biochim Biophys Acta 1978; 542:28-38.
![]()
29. Varma SD, Chand D, Sharma YR, Kuck JF Jr, Richards RD. Oxidative
stress on lens and cataract formation: role of light and oxygen. Curr
Eye Res 1984; 3:35-57. ![]()
30. Varma SD, Ets TK, Richards RD. Protection against superoxide radicals in rat lens. Ophthalmic Res 1977; 9:421-31.
31. Sawada Y, Yamazaki I. One-electron transfer reactions in
biochemical systems. 8. Kinetic study of superoxide dismutase. Biochim
Biophys Acta 1973; 327:257-65. ![]()
32. Petrat F, Bramey T, Kirsch M, De Groot H. Initiation of a
superoxide-dependent chain oxidation of lactate dehydrogenase-bound NADH
by oxidants of low and high reactivity. Free Radic Res 2005; 39:1043-57.
![]()
33. Eaton JW. Is the lens canned? Free Radic Biol Med 1991;
11:207-13. ![]()
34. Wistow GJ. Molecular biology and evolution of crystallins: gene recruitment and multifunctional proteins in the eye lens. Austin (TX): RG Landes; 1995.
35. Wistow GJ, Mulders JW, de Jong WW. The enzyme lactate
dehydrogenase as a structural protein in avian and crocodilian lenses.
Nature 1987; 326:622-4. ![]()
36. Jimenez-Asensio J, Gonzalez P, Zigler JS Jr, Garland DL.
Glyceraldehyde 3-phosphate dehydrogenase is an enzyme-crystallin in
diurnal geckos of the genus Phelsuma. Biochem Biophys Res Commun 1995;
209:796-802. ![]()
37. Graham C, Hodin J, Wistow G. A retinaldehyde dehydrogenase as a
structural protein in a mammalian eye lens. Gene recruitment of
eta-crystallin. J Biol Chem 1996; 271:15623-8. ![]()
38. Rao PV, Krishna CM, Zigler JS Jr. Identification and
characterization of the enzymatic activity of zeta-crystallin from
guinea pig lens. A novel NADPH:quinone oxidoreductase. J Biol Chem 1992;
267:96-102. ![]()
39. Hyndman D, Bauman DR, Heredia VV, Penning TM. The aldo-keto
reductase superfamily homepage. Chem Biol Interact 2003; 143-144:621-31.
![]()
40. Burczynski ME, Sridhar GR, Palackal NT, Penning TM. The reactive
oxygen species--and Michael acceptor-inducible human aldo-keto reductase
AKR1C1 reduces the alpha,beta-unsaturated aldehyde 4-hydroxy-2-nonenal
to 1,4-dihydroxy-2-nonene. J Biol Chem 2001; 276:2890-7.
![]()
41. Srivastava S, Spite M, Trent JO, West MB, Ahmed Y, Bhatnagar A.
Aldose reductase-catalyzed reduction of aldehyde phospholipids. J Biol
Chem 2004; 279:53395-406. ![]()
42. Friedburg D. Enzyme activity patterns in clear human lenses and in different types of human senile cataract. In: The human lens-in relation to cataract. New York: Elsevier; 1973. p. 117-33.
43. King G, Holmes R. Human corneal and lens aldehyde dehydrogenases.
Purification and properties of human lens ALDH1 and differential
expression as major soluble proteins in human lens (ALDH1) and cornea
(ALDH3). Adv Exp Med Biol 1997; 414:19-27. ![]()
44. King G, Holmes R. Human ocular aldehyde dehydrogenase isozymes:
distribution and properties as major soluble proteins in cornea and
lens. J Exp Zool 1998; 282:12-7. ![]()
45. King G, Hirst L, Holmes R. Human corneal and lens aldehyde
dehydrogenases. Localization and function(s) of ocular ALDH1 and ALDH3
isozymes. Adv Exp Med Biol 1999; 463:189-98. ![]()
46. Choudhary S, Xiao T, Vergara LA, Srivastava S, Nees D,
Piatigorsky J, Ansari NH. Role of aldehyde dehydrogenase isozymes in the
defense of rat lens and human lens epithelial cells against oxidative
stress. Invest Ophthalmol Vis Sci 2005; 46:259-67. ![]()
47. Wood AM, Truscott RJ. UV filters in human lenses: tryptophan
catabolism. Exp Eye Res 1993; 56:317-25. ![]()
48. Bova LM, Sweeney MH, Jamie JF, Truscott RJ. Major changes in
human ocular UV protection with age. Invest Ophthalmol Vis Sci 2001;
42:200-5. ![]()
49. Thiagarajan G, Shirao E, Ando K, Inoue A, Balasubramanian D. Role
of xanthurenic acid 8-O-beta-D-glucoside, a novel fluorophore that
accumulates in the brunescent human eye lens. Photochem Photobiol 2002;
76:368-72. ![]()
50. Inoue A, Sasaki D, Satoh K. Minimization of photooxidative insult
to calf lens protein irradiated with near UV-light in the presence of
pigmented glucosides derived from human lens protein. Exp Eye Res 2004;
79:833-7. ![]()