Received 6 January 2005 | Accepted 6 June 2005 | Published 16 June 2005
Reprint
Received 6 January 2005 | Accepted 6 June 2005 | Published 16 June 2005 |
Download Reprint |
Neutrophils promote experimental choroidal neovascularization
Jiehao Zhou,1 Lucia Pham,1 Ning Zhang,1,2
Shikun He,1,3,4 Maria-Andreea Gamulescu,1,5 Christine
Spee,1,3 Stephen J. Ryan,1,3 David
R. Hinton1,3,4
1The Arnold and Mabel Beckman Macular Research Center at the Doheny Eye Institute, 2Department of Pharmaceutical Sciences, School of Pharmacy, Departments of 3Ophthalmology, and 4Pathology, Keck School of Medicine, University of Southern California, Los Angeles, CA; 5University Eye Hospital, Regensburg, Germany
Correspondence to: David R. Hinton, MD, Departments of Pathology and Ophthalmology, Keck School of Medicine, University of Southern California, The Arnold and Mabel Beckman Macular Research Center at the Doheny Eye Institute, 2011 Zonal Avenue, HMR 209, Los Angeles, CA, 90033; Phone: (323) 442-6617; FAX: (323) 442-6688; email: dhinton@usc.edu
Abstract
Purpose: To investigate the role of neutrophils in the development of laser induced experimental choroidal neovascularization (CNV).
Methods: CNV was induced by laser photocoagulation in adult male C57BL/6J mice. Neutrophil infiltration was evaluated by histology and confocal immunohistology. The expression of neutrophil chemotactic chemokines in the regions of laser injury was determined by quantitative real-time PCR. Animals were treated with NIMP-R14, an anti-murine neutrophil monoclonal antibody (mAb), intraperitoneally to deplete neutrophils. The specific neutrophil depletion was confirmed by flow cytometry. The CNV responses were compared between neutropenic and untreated control mice on the basis of fluorescein angiography (FA), CNV lesion volume and lesion histology, and vascular endothelial growth factor (VEGF) expression by ELISA. Expression of VEGF and Angiopoietin-1 and Angiopoietin-2 protein by murine neutrophils was evaluated by confocal immunohistochemistry.
Results: Neutrophils infiltrated the sites of laser injury as early as day 1 after laser treatment and peaked at day 3. The neutrophil infiltration correlated with enhanced mRNA expression of neutrophil chemotactic chemokines MIP-2 and KC in the lesions. Administration of NIMP-R14 mAb specifically depleted neutrophils. Analysis of FA, CNV volume, and lesion histology, all demonstrated a moderate decrease in the CNV response in neutropenic mice compared to control mice (p<0.01). The reduction in the CNV response in neutropenic mice was associated with decreased VEGF protein levels in the ocular posterior segment. Murine neutrophils contained VEGF and Angiopoietin-1 and Angiopoietin-2 proteins.
Conclusions: Neutrophil invasion was part of early inflammatory responses during laser induced CNV. Neutrophil depletion correlated with reduced CNV responses and decreased VEGF protein expression. These data suggest that neutrophils promoted the early development of CNV possibly via secretion of angiogenic growth factors.
Introduction
Choroidal neovascularization (CNV) is the growth of new blood vessels from existing choroidal vessels into the subretinal space [1-3]. CNV is a common complication of age-related macular degeneration (AMD) and represents the most important cause of severe visual loss in this disorder [3-7]. Considerable efforts are now being made to develop efficacious therapies for CNV based upon advances in our understanding of CNV pathogenesis in human patients and in murine and primate CNV models [6].
It has been proposed that in the retina and choroid, the balance between pro-angiogenic and anti-angiogenic factors is under tight control in order to prevent pathologic angiogenesis [8], and that an increase in pro-angiogenic and a decrease in anti-angiogenic factors may be critical for the development of CNV [9,10]. Recently, inflammatory processes including immune complex deposition, complement activation and macrophage infiltration have been proposed as important mediators of CNV pathogenesis [11-13]. Clinical trials with anti-inflammatory steroids have shown a favorable effect on patients with neovascular AMD further suggesting that inflammation is important in the pathogenesis of CNV [14,15].
Neutrophils are the dominant infiltrating leukocyte population in early stages of inflammation, and they can initiate a complex cascade that leads to a complete inflammatory response [16,17]. Several lines of evidence have suggested that neutrophils are also potent initiators of angiogenesis, in part related to their ability to release a potent group of preformed pro-angiogenic factors including vascular endothelial growth factor (VEGF) [18-23]. The possibility that neutrophils could play a role in human choroidal disease is supported by the finding of increased number of neutrophils in the neovascular choriocapillaris of patients with diabetes and in the choriocapillaris of streptozotocin-induced experimental diabetes [24-26]. Although neutrophil infiltration has not been reported in postmortem donor eyes or submacular surgical specimens obtained from patients with neovascular AMD, such specimens are obtained relatively late in the course of disease when neutrophils are unlikely to be found [13,27].
Animal models of CNV represent one of the most important tools for elucidating the mechanisms involved in the pathogenesis of CNV and to test the potential of anti-angiogenic drugs. At this time, the laser induced Bruch's membrane photocoagulation model is the most widely accepted and most frequently utilized model of CNV [28]. Neutrophils were reported to be present in early stages of laser-induced CNV lesions in nonhuman primates over 10 years ago [29,30]. More recently, neutrophil infiltration was also noted in the early stages of laser induced CNV in the mouse [31]. There is a critical need to understand the role of neutrophils in experimental laser induced CNV since this model is being used in translational studies designed to test the preclinical potential of novel CNV therapies.
In the present study, we evaluated the role of neutrophils in laser induced murine CNV by determining the kinetics of neutrophil infiltration into the site of laser injury and the effect of neutrophil depletion on disease pathogenesis. Laser treatment in neutropenic animals correlated with a reduced CNV response and decreased levels of VEGF expression.
Methods
Animals
C57BL/6 male mice were purchased from the National Cancer Institute (Frederick, MD). Mice between 6 to 8 weeks old were fed standard laboratory chow in an air-conditioned room, and a 12 h light 12 h dark cycle were used. All procedures were performed in compliance with Keck School of Medicine Institutional Animal Care and Use Committee approved protocols and ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. For all surgical procedures, mice were anesthetized with ketamine (80 mg/kg) and xylazine (8 mg/kg), and pupils were dilated with topical 1% tropicamide (Alcon, Fort Worth, TX).
Laser photocoagulation
Diode laser photocoagulation (75 μm spot size, 0.1 s duration, 140 mW) was performed on both eyes of each mouse on day 0 [32]. For the purpose of CNV FA, CNV volume, and histology analysis, two laser photocoagulation burns were delivered to the retina, lateral to the optic disc through a slit lamp using a coverglass as a contact lens. For the purpose of RNA isolation, four discrete lesions were generated. Only lesions in which a subretinal bubble or focal serous detachment of the retina developed were used for experiments.
Histology
For histopathologic analysis, eyes were enucleated and the anterior poles and neurosensory retina were removed. The remaining eye cups containing RPE-choroid-sclera complex were snap-frozen in tissue freezing medium (Triangle Biomedical Sciences, Durham, NC). Frozen sections (6 μm) were fixed with 100% acetone at room temperature, air dried and stained with hematoxylin and eosin (H &E) to assess the histology of the laser lesion and subsequent CNV development. Distribution of neutrophils was examined in frozen sections using anti-Ly-6G mAb (1A8; BD PharMingen, San Diego, CA) as the primary antibody (Ab) and goat anti-rat as a secondary Ab (Vector Laboratory, Burlingame, CA) [32]. Briefly, frozen sections were fixed for 10 min in acetone and re-hydrated in PBS for 10 min. Following overnight incubation with primary mAb (1:100 dilution) at 4 °C, sections were incubated with the biotinylated secondary Ab (1:400 dilution) for 1 h at room temperature (RT). Visualization was achieved via an avidin-biotin peroxidase complex using AEC as chromogen (Vectastain-ABC kit; Vector Laboratories, Burlingame, CA). Control sections were incubated with isotype-matched primary mAb. All slides were examined in a masked fashion.
RNA Isolation and real-time PCR
The retinal pigment epithelium (RPE) and choroid immediately around the laser spots, and in later stages including the CNV itself, were dissected from both eyes of 5 animals at each time point after laser application. Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. The samples from the same time points were pooled. Each cDNA sample was generated and amplified using MessageAmpTM (Ambion). Semiquantitative real-time PCR analysis was conducted with the ABI 5700 SDS (Applied Biosystems, Foster City, CA) using SYBR green. Each cDNA sample was analyzed in duplicate [33]. The sequence of the primer pairs are: L-32 5'-TGG TTT TCT TGT TGC TCC CAT A-3' and 5'-GGG TGC GGA GAA GGT TCA A-3'; MIP-2 5'-CCT GCC AAG GGT TGA CTT CA-3' and 5'-TTC TGT CTG GGC GCA GTG-3'; KC 5'-ACA GTC CCG CTG ACC AAG AG-3' and 5'-CAC TGA CAG CGC AGC TCA TT-3'. The linearity of each primer pair was confirmed to have a correlation coefficient of >0.98 by measuring 5 fold serial dilution curves with cDNA samples. The Ct value is defined as the cycle number at which fluorescence exceeds a threshold value of 0.5. Expression levels for mRNA were normalized to the housekeeping gene L32 and converted to a linearized value using the following formula [34]:
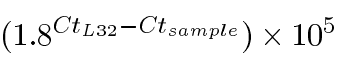
Neutrophil depletion
Neutrophils were depleted by intraperitoneal (ip) administration of 250 μg/injection of NIMP-R14 mAb, an anti-mouse neutrophil mAb, in 0.5 ml of sterile PBS at days -1, +1, and +3 relative to laser photocoagulation [35,36]. At same time points, mice received 250 μg/injection of rat IgG2b (an isotype control mAb) or 0.5 ml of sterile PBS as controls. Our data demonstrated that there was no difference in laser induced CNV response between isotype control mAb treated animals and PBS treated animals (see the Results section). The depletion of neutrophils was confirmed by the flow cytometric analysis of dissociated spleen cells [37].
Flow cytometry
Surface marker expression was determined on cells isolated from spleens by staining with mAbs specific for Ly-6G (1A8, BD Pharmingen), and F4/80 (Cl:A3-1, Serotech, Raleigh, NC). Nonspecific binding was minimized by blocking with purified rat anti-mouse FcIII/IIR mAb (2.4G2, BD Pharmingen) and naive mouse serum in PBS containing 0.5% bovine serum albumin (Sigma, Saint Louis, MO) for 10 min prior to staining. Cells were stained for 30 min on ice, washed twice with PBS containing 0.5% bovine serum albumin, and analyzed using a FACStar flow cytometer and Cellquest Pro software (version 4.0.2 Becton Dickinson, Mountain View, CA).
Fluorescein angiography
The effects of neutrophil depletion on the development of CNV were evaluated by semiquantitative assessment of late phase fluorescein angiograms. Mice were anesthetized with ketamine and xylazine and pupils were fully dilated with topical 1% tropicamide. Images were captured using a 35 mm Kowa hand-held fundus camera, (Genesis, Tokyo, Japan) 3 min after intraperitoneal injection of 0.1 mL of 2.5% fluorescein sodium (Akorn, Decatur, IL), as described [38]. Leakage was defined as the presence of a hyperfluorescent lesion that increased in size with time in the late-phase angiogram. Angiography was graded in a masked fashion by two examiners using reference angiograms. Angiograms were graded as follows: 0, no leakage; 1, slight leakage; 2, moderate leakage; 3, prominent leakage.
Choroidal flat mount preparation
Eyes were enucleated at various time points after laser photocoagulation and fixed with 2% paraformaldehyde for 1 h at 4 °C. Eye cups were obtained by removing anterior poles and neurosensory retina, and washed in PBS for 3 times. The remaining eye cups containing RPE-choroid-sclera complex were incubated with blocking buffer (PBS containing 1% bovine serum albumin and 0.5% Triton X-100) for 1 h at room temperature followed by overnight incubation with appropriate chromaphore conjugated mAb in blocking buffer at 4 °C. After 4 washings with PBS containing 0.1% Triton X-100, the flat mount of the RPE-choroid-sclera complex was made by 4 relaxing radial incisions and mounted in the VECTASHIELD mounting medium (Vector Laboratories).
Quantitation of inflammatory cell infiltration and CNV volume by confocal microscopy
Fluorescence volume measurements were accomplished by creating image stacks of optical slices within lesions. Flat mounts stained with 1 mg/ml FITC-Ly-6G, FITC-F4/80, or 10 mg/ml FITC-isolectin B4 [38] were visualized using the 20x objective of a scanning LSM510 confocal microscope (Zeiss, Germany). The image stacks were generated in the Z plane with the confocal microscope set to excite at 488 nm and detect at 505 to 530 nms. Images were further processed using the LSM software (version 2.8 SP1) by closely circumscribing and digitally extracting the fluorescent lesion areas throughout the entire image stack. The extracted lesion was processed through the LSM topography software in order to generate a digital topographical image representation of the lesion and an image volume. The topographical analysis program determines and displays the objects surface contours by detecting fluorescent signal from the top of the imagine stack and then measures everything under the surface to yield a final volume (μm3), which reflects the quantity of the infiltrating neutrophils, macrophages, or CNV fluorescence volume.
VEGF ELISA
The posterior pole of individual eyes from mice were homogenized in 250 μl buffer (80 mM Tris-HCL, 4 mM MgCl2, 0.5 mM phenylmethylsulfonylfluoride) containing mixed protease inhibitors (Roche, Basel, Switzerland) for protein extraction at day 3 and 7 after laser treatment (n=5). One eye from each of the five untreated mice served as controls. Protein homogenates were centrifuged at 14,000x g for 10 min to remove tissue debris. Samples were treated with 10 units of DNase I (Roche) for 20 min at 37 °C to release protein bound to DNA. Total protein concentration was determined by Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). VEGF protein expression was assessed with 30 μg total protein per sample in triplicate by a VEGF ELISA kit (R&D Systems, Minneapolis, MN). The detection range of this assay is 3.0-500 pg/ml VEGF.
Isolation of neutrophil enriched population and VEGF, Angiopoietin-1 and Angiopoietin-2 staining
A neutrophil enriched population was isolated from murine peritoneal exudates after glycogen challenge [39,40]. Briefly, oyster glycogen (Sigma) was dissolved in sterile 0.9% NaCl at a concentration of 1 mg/ml. A total of 1 ml was injected ip into male C57BL/6 mice, and the peritoneal exudates were harvested 4 h later. The exudate cells were approximately 90% neutrophils determined by characteristic morphology. The neutrophil enriched population was centrifuged onto superfrost plus micro slides (VWR Scientific, West Chester, PA). For confocal microscopy, slides with neutrophils were fixed in acetone for 10 min at room temperature. The expression of VEGF was detected with goat anti-mouse VEGF (R&D) as primary Ab and Rhodamine-conjugated rabbit anti-goat (Chemicon, Temecula, CA), as secondary Ab. Similarly, the expression of angiopoietin (Ang)-1 and Ang-2 were detected with goat anti-human Ang-1 and Ang-2 (Santa Cruz Biotechnology, Santa Cruz CA) as primary Ab and Rhodamine-conjugated rabbit anti-goat (Chemicon, Temecula, CA), as secondary Ab. The slides were mounted in the VECTASHIELD mounting medium for the fluorescence with DAPI (Vector Laboratories) and examined using a Zeiss LSM510 confocal microscope.
Statistics
Statistical analysis of FA score was performed using the Wilcoxon rank sum test. Values were reported as median of each group. All other statistical analyses were performed using Student's t-test. Values reported in figures represent mean and standard deviation. Accepted level of significance for all tests was <0.05.
Results
Early neutrophil responses in laser-induced CNV
The role of neutrophils in laser induced CNV was initially examined by immunohistochemistry with anti-Ly-6G, a specific mAb recognizing murine neutrophils, at different time points after laser injury. Prominent neutrophil infiltration into lesion sites was observed at day 3 after laser photocoagulation (Figure 1B). No positive staining was observed on slides stained with rat IgG2b, an isotype control of Ly-6G, confirming the staining specificity (Figure 1A). The presence of neutrophils within the laser lesions was also confirmed by their characteristic multilobulated nuclear morphology (Figure 1C). Furthermore, the kinetics of neutrophil influx to the site of laser injury was determined via quantitative analysis of fluorescent immunostaining of RPE-Choroidal flat mounts with Ly-6G. No neutrophils could be detected in the RPE-Choroidal complex from untreated eyes. However, neutrophils rapidly infiltrated into the lesions within 1 day after laser injury (Figure 1D). The accumulation of neutrophils within the lesions peaked at day 3 and declined at day 6 following laser photocoagulation (Figure 1D).
Neutrophil infiltration during inflammation usually results from the increased expression of neutrophil chemotactic chemokines [41]. The expression of both MIP-2 and KC mRNA, which are known as neutrophil specific chemoattractants [42], were found in the region of the laser lesions. Neither MIP-2 nor KC mRNA was detectable in the eyes from the untreated animals (Figure 2). The expression of both MIP-2 and KC mRNA was induced following laser treatment as early as 6 h after injury. The peak expression of MIP-2 and KC mRNA was found at 12 h and 6 h after laser treatment, respectively (Figure 2). The expression of MIP-2 and KC mRNA declined rapidly and were below the limit of detection 3 days following laser injury (Figure 2). These data were consistent with early neutrophil infiltration into the site of laser injury in response to increased MIP-2/KC expression.
NIMP-R14 treatment specifically deplete neutrophils in vivo
To determine the role of neutrophils in the development of laser-induced CNV, neutrophils were systemically depleted by ip administration of NIMP-R14 [35,36]. Neutrophil depletion was confirmed by flow cytometry via staining of cells isolated from mouse spleen with Ly-6G (Figure 3A). Among the splenocytes from control mice about 4% were positive for Ly-6G. By contrast, NIMP-R14 treatment dramatically reduced the percentage of Ly-6G-positive cells to less than 0.2%, suggesting a >95% reduction of neutrophil in the population. Macrophages play a very important role in the development of laser induced CNV [38,43,44]. The effect of NIMP-R14 treatment on the macrophage population was also evaluated by flow cytometry via the staining of F4/80, a macrophage specific mAb. In contrast to the large reduction of neutrophil population, NIMP-R14 treatment did not deplete macrophages (Figure 3B).
Neutropenia and reduced CNV responses
Treatment with NIMP-R14 not only depleted neutrophils in the periphery but also at sites of laser lesions. The absence of neutrophils within lesions was demonstrated in NIMP-R14 treated mice via quantitative confocal immunohistochemistry of RPE-choroidal flat mounts with Ly-6G (Figure 4A). By contrast, macrophage infiltration into the subretinal region of laser lesions was not affected by neutrophil depletion (Figure 4B).
The effect of neutrophil depletion on the development of laser induced CNV was evaluated by FA, CNV volume quantitation, and histopathological analysis. Fluorescein angiograms of both eyes in each animal were evaluated according to the grading system described in the Methods. Representative examples of the angiographic appearance in neutropenic and control mice are shown in (Figure 4C). Scoring of fluorescein angiograms revealed that neutropenic mice exhibited approximately 33% reduction in fluorescein leakage compared to controls at 1 week after laser photocoagulation (Figure 4E). However, no difference was obtained in the frequency of individual lesion formation with fluorescein leakage between the neutropenic and control groups. A choroidal flat mount analysis with isolectin B4 demonstrated decreased CNV lesion size in the neutropenic mice compared to controls (Figure 4D). Quantitative measurement of the area of CNV showed that the neutrophil depletion resulted in approximately 43% decrease in CNV vascular volume compared to control mice at 1 week after laser photocoagulation (Figure 4F). Histopathological studies also confirmed that neutropenic mice showed a reduction in lesion diameter and thickness (Figure 4G). No difference of CNV responses was observed at 2 weeks after laser photocoagulation between neutropenic and control mice. These data suggest that the neutrophil depletion moderately attenuated CNV severity in early stages, although it did not prevent CNV formation.
Neutropenia and reduced VEGF expression
VEGF exerts a critical pathogenic role in choroidal angiogenesis [45]. VEGF protein levels in the posterior poles were examined to determine whether neutrophil depletion altered the expression of VEGF. Laser treatment greatly increased VEGF expression in the posterior poles of eyes of PBS-treated control mice compared to animals without laser at day 3 and day 7 (Figure 5). Neutropil depletion significantly decreased VEGF protein levels by 25% compared to control mice on day 3 after laser treatment (Figure 5). However, there was no difference in VEGF expression between neutropenic and mice treated with PBS on day 7 after laser treatment. Confocal microscopy revealed that neutrophils from C57BL/6 mice contained several pro-angiogenic factors including VEGF, Ang-1 and Ang-2 (Figure 6). These data suggested that neutrophils may contribute to neovascularization by directly releasing pro-angiogenic proteins at the early stage of CNV development.
Discussion
Neutrophils are the most abundant leukocyte in the blood circulation that can rapidly infiltrate an injured site during inflammation. The present study demonstrated that prompt neutrophil infiltration was associated with rapid upregulation of MIP-2 and KC mRNA suggesting that these chemokines are the likely neutrophil chemoattractants in this model. Both MIP-2 and KC are murine counterparts of human neutrophil chemokine IL-8 [42]. MIP-2, KC, and IL-8 exert their chemoattractant function via interaction with their cognate receptor CXCR2 [46]. Enhanced IL-8 expression has been demonstrated in human ocular neovascular disorders [47]. Depletion of IL-8 is associated with inhibition of tumor-associated angiogenesis [48]. Wound healing in CXCR2-deficient mice shows delayed neovascularization associated with decreased neutrophil recruitment [19]. It has been reported that RPE cells secrete IL-8 in response to various stimuli including proinflammatory cytokines IL-1b or TNF-α, and leukocyte/RPE cell-cell contact through a phosphoinositide 3-kinase/Akt dependent pathway [49]. It is unlikely that cell-cell contact induced the initial neutrophil chemokine expression in this model because neutrophil infiltration was a very early event after laser injury compared to other leukocytes including macrophages and T cells. By contrast, our preliminary data shows that expression of both IL-1b and TNF-α mRNA were upregulated 6 h after the laser treatment (data not shown). Thus, IL-1b or TNF-α produced by activated RPE cells may induce MIP-2/KC expression via an autocrine or paracrine manner.
In our laser induced model, there was a lag time between the peak expression of MIP-2/KC chemokine mRNA and the maximal recruitment of neutrophils: Chemokine expression peaked 6 to 12 h after laser treatment, whereas neutrophil infiltration peaked at 72 h. While neutrophil infiltration has been shown to be coincident with MIP-2/KC expression in several animal models involving infectious agents [50], other models show a delay in neutrophil infiltration similar to our findings. In a mouse model of pneumococcal pneumonia, MIP-2/KC expression precedes pulmonary neutrophil recruitment by more than 36 h [51]. In a more relevant light induced inflammation model, Gollnick et al. [52] report that maximal MIP-2/KC expression is at 6 h and the peak neutrophil recruitment is not observed until between 24 and 48 h. A similar phenomenon has been observed in a carrageenan-induced inflammatory response [53]. Therefore, it is proposed that the lag time between neutrophil infiltration and chemokine expression is probably determined by the intrinsic characteristics of laser injury within the outer retinal microenvironment.
In contrast to the traditional notion that neutrophils are terminally differentiated, short-lived phagocytes, recent evidence indicates that neutrophils are remarkably multifunctional cells. Neutrophils entering tissue possess a longer life span and can be activated to perform various functions associated with both innate immunity and adaptive immune responses including phagocytosis, antigen presentation, and immunoregulation [54,55]. In this model, neutrophils appear to promote angiogenesis in the subretinal space. One underlying mechanism for the pro-angiogenic activity of neutrophils is possibly due to the fact that neutrophil granules contain a variety of preformed pro-angiogenic proteins [18-23]. Compared to other pro-angiogenic cytokines and growth factors, VEGF is probably the most important soluble pro-angiogenic factor and the inhibition of VEGF activity is associated with reduced CNV response in the laser induced CNV [56]. The expression of VEGF protein in specific granules of human neutrophils has been demonstrated [18] and now we confirm its expression in C57BL/6 murine neutrophils. During the early stage of laser induced CNV, infiltrating neutrophils might secrete preformed VEGF into the subretinal space upon activation by proinflammatory cytokines including TNF-α and IL-1b [18,57]. Therefore, the initial VEGF expression in the subretinal space during laser-induced CNV might directly derive from infiltrating neutrophils. This hypothesis was supported by the observation that depletion of neutrophils was associated with reduced VEGF protein expression at day 3 after laser injury (Figure 5). An alternative explanation is that the direct or indirect interactions between infiltrating neutrophils and RPE enhance VEGF production from RPE.
Choroidal angiogenesis is associated with the expression of multiple growth factors in addition to VEGF, and in this study, we showed that C57BL/6 neutrophils also contain Ang-1 and Ang-2. Ang-1 and Ang-2 are expressed in human CNV membranes [58,59], and inhibition of angiopoietin signaling inhibits the development of experimental laser induced CNV [60]. A recent study indicates that both Ang-1 and Ang-2 induce neutrophil adhesion, providing further support for crosstalk between neutrophils and angiogenic factors [61].
Neutrophils secrete matrix metalloproteinase-9 (MMP-9) [62] which can increase the bioavailability of VEGF [63,64]. Lambert et al. [31] demonstrated that CNV development is reduced in MMP-9 deficient mice. In the same study, MMP-9 protein expression was detected exclusively at the site of laser-induced injury by immunohistochemistry. MMP-9 staining intensity is observed only at the early stage of CNV and peaks at day 3 after laser injury. Furthermore, no expression of MMP-9 mRNA is detectable using in situ hybridization despite of the upregulation of MMP-9 protein [65]. It has been established that neutrophils contain preformed MMP-9 protein in their intracellular granules and release MMP-9 upon stimulation [66]. Our present study suggested that the localization and kinetics of neutrophils correlated with those of MMP-9 protein expression during CNV. Combined together, these studies suggest that neutrophils are most likely an initial provider of MMP-9 in this model.
In the present study, a moderate reduction (approximately 33-43%) in CNV was observed by neutrophil depletion. Multiple cellular and soluble factors are involved in the development of CNV. One of the important cellular components influencing CNV development is the macrophage, which also has the capacity to secrete VEGF. The depletion of neutrophils by administration of mAb NIMP-R14 neither affected the peripheral macrophage population, as demonstrated by flow cytometry, nor prevented macrophage infiltration into the sites of laser injury, as demonstrated by quantitative analysis. Neutrophil depletion was associated with a 25% reduction of VEGF protein in the posterior segment 3 days after laser injury, suggesting cells other than neutrophils contribute significantly to VEGF production in this model. The fact that there was no reduction in VEGF protein in neutropenic mice at day 7 after laser injury suggested involvement of other angiogenic mechanisms including macrophage infiltration and VEGF production from retinal resident cells such as RPE [38,67,68].
Recently, Tsutsumi-Miyahara and colleagues [69] also investigated the role of neutrophils in experimental CNV. Similar to our study, infiltration of neutrophils was demonstrated in the early phase of CNV formation. However, in their study there was no significant difference obtained between neutropenic mice and untreated controls, although neutropenic mice demonstrated a trend toward reduced CNV lesion size. The discrepancy between these two studies may result from the method used to deplete neutrophils. In our study neutrophils were depleted by 3 administrations of NIMP-R14 mAb; in contrast, they depleted neutrophils with 4 doses of the mAb, Gr-1. Gr-1 was initially shown to specifically recognize neutrophils and has been commonly used to deplete neutrophils in vivo. However, recent studies have indicated that administration of Gr-1 can also eliminate a memory-type CD8+ T cell subset with cytotoxicity and IFN-γ-producing activity [70]. Therefore, extended treatment of Gr-1 may not only deplete neutrophils but also cytotoxic CD8+ T cells. It has been proposed that cytotoxic CD8+ T cells contribute to the regression of neovascularization, possibly through Fas ligand mediated endothelial apoptosis [71,72]. It is conceivable that depletion of anti-angiogenic CD8+ T cells may partially counteract the depletion of pro-angiogenic neutrophils in experimental CNV.
In summary, neutrophils rapidly infiltrated into the subretinal regions subsequent to an increase in expression of MIP-2/KC following laser photocoagulation. In addition to their pro-inflammatory roles, neutrophils may promote the development of laser induced CNV at the early stage by providing preformed pro-angiogenic factors including VEGF, Ang-1, Ang-2, and MMP-9. These data suggest that pharmacologic targeting of neutrophils or neutrophil chemokines may be a means of inhibiting early events involved in the pathogenesis of CNV.
Acknowledgements
We thank Ernesto Barron, Xiaoping Wang, and Anthony Rodriguez for their excellent technical assistance and Laurie LaBree for statistical analysis. This study is supported by The Arnold and Mabel Beckman Foundation, Research to Prevent Blindness (Doheny Eye Institute), and National Institute of Health grants R01EY01545 and P30EY03040. M-A. Gamulescu is supported by a grant from the Deutsche Akademie der Naturforscher Leopoldina (BMBF-LPD 9901/8-52), Federal Republic of Germany.
References
1. Schneider S, Greven CM, Green WR. Photocoagulation of well-defined
choroidal neovascularization in age-related macular degeneration:
clinicopathologic correlation. Retina 1998; 18:242-50. ![]()
2. Green WR, Enger C. Age-related macular degeneration
histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture.
Ophthalmology 1993; 100:1519-35. ![]()
3. Lee P, Wang CC, Adamis AP. Ocular neovascularization: an
epidemiologic review. Surv Ophthalmol 1998; 43:245-69. ![]()
4. Argon laser photocoagulation for neovascular maculopathy.
Five-year results from randomized clinical trials. Macular
Photocoagulation Study Group. Arch Ophthalmol 1991; 109:1109-14. Erratum
in: Arch Ophthalmol 1992; 110:761. ![]()
5. Kini MM, Leibowitz HM, Colton T, Nickerson RJ, Ganley J, Dawber
TR. Prevalence of senile cataract, diabetic retinopathy, senile macular
degeneration, and open-angle glaucoma in the Framingham eye study. Am J
Ophthalmol 1978; 85:28-34. ![]()
6. Ciulla TA, Danis RP, Harris A. Age-related macular degeneration: a
review of experimental treatments. Surv Ophthalmol 1998; 43:134-46.
![]()
7. Bressler NM, Bressler SB, West SK, Fine SL, Taylor HR. The grading
and prevalence of macular degeneration in Chesapeake Bay watermen. Arch
Ophthalmol 1989; 107:847-52. ![]()
8. Das A, McGuire PG. Retinal and choroidal angiogenesis:
pathophysiology and strategies for inhibition. Prog Retin Eye Res 2003;
22:721-48. ![]()
9. Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related
macular degeneration: etiology, pathogenesis, and therapeutic
strategies. Surv Ophthalmol 2003; 48:257-93. ![]()
10. Bainbridge JW, Mistry AR, Thrasher AJ, Ali RR. Gene therapy for
ocular angiogenesis. Clin Sci (Lond) 2003; 104:561-75.
![]()
11. Johnson LV, Ozaki S, Staples MK, Erickson PA, Anderson DH. A
potential role for immune complex pathogenesis in drusen formation. Exp
Eye Res 2000; 70:441-9. ![]()
12. Killingsworth MC, Sarks JP, Sarks SH. Macrophages related to
Bruch's membrane in age-related macular degeneration. Eye 1990;
4:613-21. ![]()
13. Penfold PL, Killingsworth MC, Sarks SH. Senile macular
degeneration: the involvement of immunocompetent cells. Graefes Arch
Clin Exp Ophthalmol 1985; 223:69-76. ![]()
14. Rechtman E, Danis RP, Pratt LM, Harris A. Intravitreal
triamcinolone with photodynamic therapy for subfoveal choroidal
neovascularisation in age related macular degeneration. Br J Ophthalmol
2004; 88:344-7. ![]()
15. Ranson NT, Danis RP, Ciulla TA, Pratt L. Intravitreal
triamcinolone in subfoveal recurrence of choroidal neovascularisation
after laser treatment in macular degeneration. Br J Ophthalmol 2002;
86:527-9. ![]()
16. Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P,
Halbwachs-Mecarelli L. Neutrophils: molecules, functions and
pathophysiological aspects. Lab Invest 2000; 80:617-53.
![]()
17. Benelli R, Albini A, Noonan D. Neutrophils and angiogenesis:
potential initiators of the angiogenic cascade. Chem Immunol Allergy
2003; 83:167-81. ![]()
18. Gaudry M, Bregerie O, Andrieu V, El Benna J, Pocidalo MA, Hakim
J. Intracellular pool of vascular endothelial growth factor in human
neutrophils. Blood 1997; 90:4153-61. ![]()
19. Devalaraja RM, Nanney LB, Du J, Qian Q, Yu Y, Devalaraja MN,
Richmond A. Delayed wound healing in CXCR2 knockout mice. J Invest
Dermatol 2000; 115:234-44. Erratum in: J Invest Dermatol 2000; 115:931.
![]()
20. McCourt M, Wang JH, Sookhai S, Redmond HP. Activated human
neutrophils release hepatocyte growth factor/scatter factor. Eur J Surg
Oncol 2001; 27:396-403. ![]()
21. Takahashi GW, Andrews DF 3rd, Lilly MB, Singer JW, Alderson MR.
Effect of granulocyte-macrophage colony-stimulating factor and
interleukin-3 on interleukin-8 production by human neutrophils and
monocytes. Blood 1993; 81:357-64. ![]()
22. Scapini P, Morini M, Tecchio C, Minghelli S, Di Carlo E,
Tanghetti E, Albini A, Lowell C, Berton G, Noonan DM, Cassatella MA.
CXCL1/macrophage inflammatory protein-2-induced angiogenesis in vivo is
mediated by neutrophil-derived vascular endothelial growth factor-A. J
Immunol 2004; 172:5034-40. ![]()
23. Scapini P, Calzetti F, Cassatella MA. On the detection of
neutrophil-derived vascular endothelial growth factor (VEGF). J Immunol
Methods 1999; 232:121-9. ![]()
24. Lutty GA, Cao J, McLeod DS. Relationship of polymorphonuclear
leukocytes to capillary dropout in the human diabetic choroid. Am J
Pathol 1997; 151:707-14. ![]()
25. Joussen AM, Poulaki V, Mitsiades N, Cai WY, Suzuma I, Pak J, Ju
ST, Rook SL, Esser P, Mitsiades CS, Kirchhof B, Adamis AP, Aiello LP.
Suppression of Fas-FasL-induced endothelial cell apoptosis prevents
diabetic blood-retinal barrier breakdown in a model of
streptozotocin-induced diabetes. FASEB J 2003; 17:76-8.
![]()
26. Cao J, McLeod S, Merges CA, Lutty GA. Choriocapillaris
degeneration and related pathologic changes in human diabetic eyes. Arch
Ophthalmol 1998; 116:589-97. ![]()
27. Grossniklaus HE, Martinez JA, Brown VB, Lambert HM, Sternberg P
Jr, Capone A Jr, Aaberg TM, Lopez PF. Immunohistochemical and
histochemical properties of surgically excised subretinal neovascular
membranes in age-related macular degeneration. Am J Ophthalmol 1992;
114:464-72. ![]()
28. Ryan SJ. Subretinal neovascularization. Natural history of an
experimental model. Arch Ophthalmol 1982; 100:1804-9. ![]()
29. Miller H, Miller B, Ishibashi T, Ryan SJ. Pathogenesis of
laser-induced choroidal subretinal neovascularization. Invest Ophthalmol
Vis Sci 1990; 31:899-908. ![]()
30. Martini B, Ryan SJ. Argon laser lesions of the retina; occurrence
and origin of macrophages. Eur J Ophthalmol 1992; 2:51-7.
![]()
31. Lambert V, Munaut C, Jost M, Noel A, Werb Z, Foidart JM, Rakic
JM. Matrix metalloproteinase-9 contributes to choroidal
neovascularization. Am J Pathol 2002; 161:1247-53. ![]()
32. Murata T, Hangai M, Ishibashi T, Spee C, Gordon EM, Anderson WF,
Hinton DR, Ryan SJ. Retrovirus-mediated gene transfer to
photocoagulation-induced choroidal neovascular membranes. Invest
Ophthalmol Vis Sci 1998; 39:2474-8. ![]()
33. Fink L, Seeger W, Ermert L, Hanze J, Stahl U, Grimminger F,
Kummer W, Bohle RM. Real-time quantitative RT-PCR after laser-assisted
cell picking. Nat Med 1998; 4:1329-33. ![]()
34. Zhou J, Marten NW, Bergmann CC, Macklin WB, Hinton DR, Stohlman
SA. Expression of matrix metalloproteinases and their tissue inhibitor
during viral encephalitis. J Virol 2005; 79:4764-73. ![]()
35. Lopez AF, Strath M, Sanderson CJ. Differentiation antigens on
mouse eosinophils and neutrophils identified by monoclonal antibodies.
Br J Haematol 1984; 57:489-94. ![]()
36. Tacchini-Cottier F, Zweifel C, Belkaid Y, Mukankundiye C, Vasei
M, Launois P, Milon G, Louis JA. An immunomodulatory function for
neutrophils during the induction of a CD4+ Th2 response in BALB/c mice
infected with Leishmania major. J Immunol 2000; 165:2628-36.
![]()
37. Zhou J, Stohlman SA, Hinton DR, Marten NW. Neutrophils promote
mononuclear cell infiltration during viral-induced encephalitis. J
Immunol 2003; 170:3331-6. ![]()
38. Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J.
Macrophage depletion inhibits experimental choroidal neovascularization.
Invest Ophthalmol Vis Sci 2003; 44:3578-85. ![]()
39. Mulligan MS, Lentsch AB, Miyasaka M, Ward PA. Cytokine and
adhesion molecule requirements for neutrophil recruitment during
glycogen-induced peritonitis. Inflamm Res 1998; 47:251-5.
![]()
40. Ikeda S, Saito H, Inoue T, Fukatsu K, Han I, Furukawa S, Matsuda
T, Hidemura A. Malnutrition impairs CD11b/CD18 expression on circulating
polymorphonuclear neutrophils and subsequent exudation into inflammatory
sites in the early phase of glycogen-induced murine peritonitis. JPEN J
Parenter Enteral Nutr 2000; 24:276-9. ![]()
41. Diab A, Abdalla H, Li HL, Shi FD, Zhu J, Hojberg B, Lindquist L,
Wretlind B, Bakhiet M, Link H. Neutralization of macrophage inflammatory
protein 2 (MIP-2) and MIP-1alpha attenuates neutrophil recruitment in
the central nervous system during experimental bacterial meningitis.
Infect Immun 1999; 67:2590-601. ![]()
42. Van Damme J, Wuyts A, Froyen G, Van Coillie E, Struyf S, Billiau
A, Proost P, Wang JM, Opdenakker G. Granulocyte chemotactic protein-2
and related CXC chemokines: from gene regulation to receptor usage. J
Leukoc Biol 1997; 62:563-9. ![]()
43. Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG,
Cousins SW. Macrophage depletion diminishes lesion size and severity in
experimental choroidal neovascularization. Invest Ophthalmol Vis Sci
2003; 44:3586-92. ![]()
44. Tsutsumi C, Sonoda KH, Egashira K, Qiao H, Hisatomi T, Nakao S,
Ishibashi M, Charo IF, Sakamoto T, Murata T, Ishibashi T. The critical
role of ocular-infiltrating macrophages in the development of choroidal
neovascularization. J Leukoc Biol 2003; 74:25-32. ![]()
45. Kwak N, Okamoto N, Wood JM, Campochiaro PA. VEGF is major
stimulator in model of choroidal neovascularization. Invest Ophthalmol
Vis Sci 2000; 41:3158-64. ![]()
46. Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY,
Buechi L, Walz A, Richmond A, Strieter RM. The CXC chemokine receptor 2,
CXCR2, is the putative receptor for ELR+ CXC chemokine-induced
angiogenic activity. J Immunol 2000; 165:5269-77. ![]()
47. Yoshida A, Yoshida S, Khalil AK, Ishibashi T, Inomata H. Role of
NF-kappaB-mediated interleukin-8 expression in intraocular
neovascularization. Invest Ophthalmol Vis Sci 1998; 39:1097-106.
![]()
48. Smith DR, Polverini PJ, Kunkel SL, Orringer MB, Whyte RI, Burdick
MD, Wilke CA, Strieter RM. Inhibition of interleukin 8 attenuates
angiogenesis in bronchogenic carcinoma. J Exp Med 1994; 179:1409-15.
![]()
49. Bian ZM, Elner SG, Yoshida A, Elner VM. Differential involvement
of phosphoinositide 3-kinase/Akt in human RPE MCP-1 and IL-8 expression.
Invest Ophthalmol Vis Sci 2004; 45:1887-96. ![]()
50. Wareing MD, Lyon AB, Lu B, Gerard C, Sarawar SR. Chemokine
expression during the development and resolution of a pulmonary
leukocyte response to influenza A virus infection in mice. J Leukoc Biol
2004; 76:886-95. ![]()
51. Fillion I, Ouellet N, Simard M, Bergeron Y, Sato S, Bergeron MG.
Role of chemokines and formyl peptides in pneumococcal pneumonia-induced
monocyte/macrophage recruitment. J Immunol 2001; 166:7353-61.
![]()
52. Gollnick SO, Evans SS, Baumann H, Owczarczak B, Maier P, Vaughan
L, Wang WC, Unger E, Henderson BW. Role of cytokines in photodynamic
therapy-induced local and systemic inflammation. Br J Cancer 2003;
88:1772-9. ![]()
53. Garcia-Ramallo E, Marques T, Prats N, Beleta J, Kunkel SL,
Godessart N. Resident cell chemokine expression serves as the major
mechanism for leukocyte recruitment during local inflammation. J Immunol
2002; 169:6467-73. ![]()
54. Moulding DA, Akgul C, Derouet M, White MR, Edwards SW. BCL-2
family expression in human neutrophils during delayed and accelerated
apoptosis. J Leukoc Biol 2001; 70:783-92. ![]()
55. Yamashiro S, Kamohara H, Wang JM, Yang D, Gong WH, Yoshimura T.
Phenotypic and functional change of cytokine-activated neutrophils:
inflammatory neutrophils are heterogeneous and enhance adaptive immune
responses. J Leukoc Biol 2001; 69:698-704. ![]()
56. Krzystolik MG, Afshari MA, Adamis AP, Gaudreault J, Gragoudas ES,
Michaud NA, Li W, Connolly E, O'Neill CA, Miller JW. Prevention of
experimental choroidal neovascularization with intravitreal
anti-vascular endothelial growth factor antibody fragment. Arch
Ophthalmol 2002; 120:338-46. ![]()
57. Ogle JD, Noel JG, Sramkoski RM, Ogle CK, Alexander JW. The
effects of cytokines, platelet activating factor, and arachidonate
metabolites on C3b receptor (CR1, CD35) expression and phagocytosis by
neutrophils. Cytokine 1990; 2:447-55. ![]()
58. Martin G, Schlunck G, Hansen LL, Agostini HT. Differential
expression of angioregulatory factors in normal and CNV-derived human
retinal pigment epithelium. Graefes Arch Clin Exp Ophthalmol 2004;
242:321-6. ![]()
59. Hangai M, Murata T, Miyawaki N, Spee C, Lim JI, He S, Hinton DR,
Ryan SJ. Angiopoietin-1 upregulation by vascular endothelial growth
factor in human retinal pigment epithelial cells. Invest Ophthalmol Vis
Sci 2001; 42:1617-25. ![]()
60. Hangai M, Moon YS, Kitaya N, Chan CK, Wu DY, Peters KG, Ryan SJ,
Hinton DR. Systemically expressed soluble Tie2 inhibits intraocular
neovascularization. Hum Gene Ther 2001; 12:1311-21. ![]()
61. Lemieux C, Maliba R, Favier J, Theoret JF, Merhi Y, Sirois MG.
Angiopoietins can directly activate endothelial cells and neutrophils to
promote proinflammatory responses. Blood 2005; 105:1523-30.
![]()
62. Schwesinger C, Yee C, Rohan RM, Joussen AM, Fernandez A, Meyer
TN, Poulaki V, Ma JJ, Redmond TM, Liu S, Adamis AP, D'Amato RJ.
Intrachoroidal neovascularization in transgenic mice overexpressing
vascular endothelial growth factor in the retinal pigment epithelium. Am
J Pathol 2001; 158:1161-72. ![]()
63. Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa
K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9
triggers the angiogenic switch during carcinogenesis. Nat Cell Biol
2000; 2:737-44. ![]()
64. Engsig MT, Chen QJ, Vu TH, Pedersen AC, Therkidsen B, Lund LR,
Henriksen K, Lenhard T, Foged NT, Werb Z, Delaisse JM. Matrix
metalloproteinase 9 and vascular endothelial growth factor are essential
for osteoclast recruitment into developing long bones. J Cell Biol 2000;
151:879-89. Erratum in: J Cell Biol 2001; 152:following 417.
![]()
65. Kvanta A, Shen WY, Sarman S, Seregard S, Steen B, Rakoczy E.
Matrix metalloproteinase (MMP) expression in experimental choroidal
neovascularization. Curr Eye Res 2000; 21:684-90. ![]()
66. Opdenakker G, Van den Steen PE, Dubois B, Nelissen I, Van Coillie
E, Masure S, Proost P, Van Damme J. Gelatinase B functions as regulator
and effector in leukocyte biology. J Leukoc Biol 2001; 69:851-9.
![]()
67. Frank RN, Amin RH, Eliott D, Puklin JE, Abrams GW. Basic
fibroblast growth factor and vascular endothelial growth factor are
present in epiretinal and choroidal neovascular membranes. Am J
Ophthalmol 1996; 122:393-403. ![]()
68. Adamis AP, Shima DT, Yeo KT, Yeo TK, Brown LF, Berse B, D'Amore
PA, Folkman J. Synthesis and secretion of vascular permeability
factor/vascular endothelial growth factor by human retinal pigment
epithelial cells. Biochem Biophys Res Commun 1993; 193:631-8.
![]()
69. Tsutsumi-Miyahara C, Sonoda KH, Egashira K, Ishibashi M, Qiao H,
Oshima T, Murata T, Miyazaki M, Charo IF, Hamano S, Ishibashi T. The
relative contributions of each subset of ocular infiltrated cells in
experimental choroidal neovascularisation. Br J Ophthalmol 2004;
88:1217-22. ![]()
70. Matsuzaki J, Tsuji T, Chamoto K, Takeshima T, Sendo F, Nishimura
T. Successful elimination of memory-type CD8+ T cell subsets by the
administration of anti-Gr-1 monoclonal antibody in vivo. Cell Immunol
2003; 224:98-105. ![]()
71. Ishida S, Yamashiro K, Usui T, Kaji Y, Ogura Y, Hida T, Honda Y,
Oguchi Y, Adamis AP. Leukocytes mediate retinal vascular remodeling
during development and vaso-obliteration in disease. Nat Med 2003;
9:781-8. ![]()
72. Ishida S, Usui T, Yamashiro K, Kaji Y, Amano S, Ogura Y, Hida T,
Oguchi Y, Ambati J, Miller JW, Gragoudas ES, Ng YS, D'Amore PA, Shima
DT, Adamis AP. VEGF164-mediated inflammation is required for
pathological, but not physiological, ischemia-induced retinal
neovascularization. J Exp Med 2003; 198:483-9. ![]()