Received 2 December 2003 | Accepted 2 December 2004 | Published 13 January 2005
Reprint
Received 2 December 2003 | Accepted 2 December 2004 | Published 13 January 2005 |
Download Reprint |
Neonatal aphakia retards ocular growth and alters scleral gene expression in rhesus monkeys
Roy W. Tarnuzzer,1 Alcides
Fernandes,2,3 P. Michael Iuvone,2,3,4 Scott R.
Lambert2,3
1Health Research Institute, Orlando Regional Healthcare, Orlando, FL; 2Division of Visual Science, Yerkes Regional Primate Research Center and the Departments of 3Ophthalmology and 4Pharmacology, Emory University, Atlanta, GA
Correspondence to: Roy W. Tarnuzzer, Ph.D., Director, Health Research Institute Orlando Regional Healthcare; 110 Bonnie Loch Court Orlando, FL, 32806; Phone: (321) 841-8410; email: rtarnuzz@orhs.org
Abstract
Purpose: We hypothesize that remodeling of the scleral extracellular matrix, involving collagen and proteoglycan synthesis and turnover, is a key process involved in ocular growth. Decreased axial elongation is observed following neonatal removal of the crystalline lens in a rhesus monkey model of congenital cataract. We wanted to determine changes in gene expression in the operated and companion eye following lensectomy, especially for extracellular matrix in the sclera.
Methods: Between 4 and 7 days of age, infant monkeys underwent surgical removal of the lens from the right eye. Axial lengths of the operated and unmanipulated fellow eyes were measured and when interocular differences of >0.4 mm were achieved, monkeys were sacrificed and RNA was isolated from sclera. In order to determine changes in scleral gene expression in aphakic versus control eyes, we used Clontech's Atlas Gene Array (Human Cancer Array version 1.2) hybridized with total RNA from three monkeys.
Results: Atlas Gene Array analysis demonstrated differential expression of several genes in the operated versus the unmanipulated eye. Most notably, there was a statistically significant increase in expression of several extracellular matrix (ECM) genes including: aggrecan, decorin, biglycan, several collagens, and tenascin in the RNA from the sclera of the aphakic eyes when compared to the unmanipulated eyes. Genes for several matrix metalloproteinases (MMPs) showed no significant change following lens removal although there was a trend towards decreased expression. There were also statistically significant changes in the pattern of gene expression in the operated eye relative to the unmanipulated eye for cell adhesion, cell cycle, apoptosis, and cytoskeleton transcripts.
Conclusions: Our results suggest that removal of the crystalline lens alters gene expression in the sclera with a prominent upregulation of ECM transcripts. These data support recent evidence that remodeling of the ECM composition of the sclera may be an important regulator of ocular growth.
Introduction
Monocular aphakia retards axial elongation in neonatal rhesus monkeys [1]. Optically correcting an aphakic eye with a contact lens or an intraocular lens did not alter the axial elongation of the aphakic eye [1]. Additionally, grating acuity tested by psychophysical methods demonstrated excellent vision in some corrected aphakic eyes [2,3]. In our model, the implication of these findings is that optical correction alone is not enough to stimulate normal ocular growth in aphakic eyes. Therefore, for this study, we utilized a simple aphakia model without vision correction.
In several other models of ocular growth, remodeling of the extracellular matrix of the sclera has been demonstrated at both the transcriptional and the post-translational levels [4-9]. The sclera is composed mostly of extracellular matrix (ECM). Scleral ECM contains proteoglycans aggrecan, biglycan, decorin, collagen type I, III, V, VI, and VIII, and elastic fibers [10]. The physical properties of the sclera generally depend on the composition and interactions of these extracellular matrix molecules. Sulfated glycosaminoglycan composition can affect the elasticity and pliability of the sclera by modulating the hydration of the sclera and cross-linking of collagen fibrils [11]. Studies of the proteoglycan composition of the human sclera indicate the presence of aggrecan, biglycan, and decorin [10]. Aggrecan has a large core protein and chondroitin and keratan sulfate glycosaminoglycan side chains that impart water-binding activity [10]. Decorin is usually associated with collagen fibrils and is involved in the organization of the extracellular matrix [12]. ECM molecules such as collagens and proteoglycans are upregulated by fibrogenic growth factors such as transforming growth factor β1 (TGFβ1) [13]. Decorin and biglycan bind TGFβ1, modifying its biological activity and helping to localize it to a specific area [14]. This suggests a mechanism by which fibrogenic growth factors such as TGFβ1 may be specifically localized to the sclera without temporal tanscriptional upregulation in the cells of the sclera.
Recent studies show that visual experience can drastically influence the synthesis and turnover of scleral ECM. Many of these studies focus on scleral extracellular matrix changes induced by form deprivation. Form deprivation induces myopia and a concomitant increase in axial length [15]. In the tree shrew, there is a correlation between vitreous chamber elongation and synthesis of ECM such as decorin and collagen type 1α1 as well as proteases and their regulators involved in matrix turnover such as MMP-2, MMP-3, and TIMP1 [8]. These observations suggest that changes in scleral extracellular matrix synthesis, accumulation, and turnover may be associated with changes in ocular growth during normal development and abnormal ocular growth.
This report investigates gene expression in the sclera of rhesus monkeys following removal of the crystalline lens. Most previous models have looked at ocular growth in relation to increasing axial length. We now report the expression profile for transcripts of genes in the sclera of aphakic eyes demonstrating significant decreases in axial elongation compared to the unmanipulated companion eyes.
Methods
Animals and surgery
Twelve newborn rhesus monkeys (Macaca mulatta) underwent lensectomy in the right eye 4 to 7 days after birth according to an established protocol [1]. One monkey, RHB-8, also had a sham operation performed on the left eye. All monkeys were born at the Yerkes National Primate Research Center breeding facility and received a complete ophthalmic examination on the day of birth, including A-scan axial length measurements. Thereafter, they were housed in individual isolates under a daily light-dark cycle of 10 h of light and 14 h of dark. Postoperative regimen included daily inspections, topical antibiotic ointment twice daily, 1% atropine sulfate solution once a day for five days, and flunixine meglumine (Banamine) intramuscularly, 1 mg/kg, four times daily for 5 days. All procedures were in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and were approved by the Institutional Animal Care and Use Committee of Emory University.
Axial length measurements
The same ophthalmologists (SRL and AF) examined all monkey eyes. Anesthesia of the monkeys was induced with an intramuscular injection of ketamine hydrochloride (10 mg/kg) and maintained with Telazol (4 mg/kg). The monkeys were placed in the supine position in a headrest apparatus to stabilize the head. Cycloplegia was induced by the instillation of 1 drop of 1% cyclopentolate hydrochloride and 1 drop of 2.5% phenylephrine hydrochloride. This procedure was repeated 2 more times at 5 min intervals. A lid speculum was used to hold the eyelids open. Corneas were kept moist with saline drops to preserve the integrity of the corneal epithelium. Axial lengths were measured with an ultrasound A-Scan system (Sonomed, Lake Success, NY). At each examination session, the A-Scan was calibrated for accuracy against a standard of known length. Tissue velocities were set at 1532 m/s for the aphakic eyes and 1550 m/s for the unmanipulated fellow eyes [16]. Care was taken to ensure that the measurements were on axis and that the ultrasound transducer did not compress the cornea. The mean of 10 measurements of each eye defined that eye's axial length.
Harvesting of tissues and RNA isolation
When intraocular differences of axial length were at least 0.4 mm, subjects received a lethal intravenous dose of pentobarbital. The eyes were enucleated and placed in cold phosphate buffered saline (PBS). The eyes were sectioned at the pars plana and the vitreous humor removed from the eyecup. The retina, RPE, and choroid were subsequently dissected away from the sclera. Scleral tissue was minced with a sterile blade and homogenized in a ground glass dounce homogenizer with 2.5 ml of Trizol reagent (Life Technologies, Gibco BRL, Gaithersberg, MD) on ice. The time from enucleation to sample homogenization was less than 5 min. The samples were centrifuged for 10 min at 10,000 rpm at 4 °C and the aqueous layer isolated and the RNA was precipitated with isopropyl alcohol. Samples were stored at -80 °C in 70% ethanol/diethyl pyrocarbonate treated water until gene array analysis. RNA concentrations and 260 nm/280 nm ratios were determined by UV spectrophotometry prior to labeling for gene array analysis.
Gene array
Scleral RNA from 3 monkeys was independently analyzed for gene expression by Atlas Gene Array (Clontech, Palo Alto, CA) differential hybridization. Four micrograms of total RNA from each operated and unmanipulated eye was labeled with 32P-ATP by reverse transcription according to the manufacturer's protocols. Atlas Human Cancer Array was used for analysis of scleral RNA from monkey RCZ6 and Human Cancer Array 1.2 was used to analyze scleral RNA from monkeys RQA7, and REK7. Differences in membranes used were due to availability of the arrays at the time the assays were performed. Gene array membranes were hybridized with 1x107 cpm of each probe at 68 °C for 20 h with constant agitation. Blots were washed 4 times in 2X SSC, 1% SDS at 68 °C for 30 min followed by a single wash in 0.1X SSC, 0.5% SDS at 68 °C for 30 min. The membranes were exposed to X-ray film for 24 to 72 h before development of the autoradiograms. Autoradiograms were scanned and saved as TIFF images and analyzed using Atlas Image version 2.01 (Clontech). Data generated by Atlas Image version 2.01 software were normalized to G3PDH signal and the output data was converted to tab delimited text and further analyzed using Atlas Navigator version 1.0 (Clontech). The Atlas Navigator software allows K-means cluster analysis which was performed and genes were grouped into a specified number of categories (K=5) based on the similarity of their expression profiles. K-means clustering uses a distance definition based on standard correlation and considers the overall "shape" of the expression pattern. The equation presented below is used to compare the expression profiles of two genes and calculate a value, R, representing the similarity of the profiles. This analysis considers the expression of genes across all arrays in a chosen experiment set and allows grouping together of genes based on their overall expression patterns in the experiment.
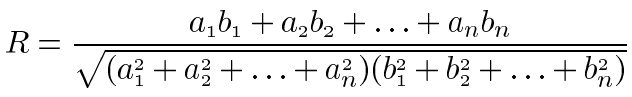
Statistical analysis
Mean values for axial length measurements from both aphakic and unmanipulated eyes (n=12) were compared using a paired Student's t-test. Mean values for gene expression for the different genes from aphakic and unmanipulated eyes were compared using a paired Student's t-test with and without the Bonferroni correction for multiple comparisons. A p value of less than 0.05 was considered statistically significant.
Results
Axial elongation measurements
All of the infant monkeys that underwent lensectomy of their right eye showed a greater than 0.4 mm difference in axial length in the operated versus the unmanipulated eye within two months of surgery. In general, both eyes continued to elongate postoperatively with the unmanipulated eye, outpacing the aphakic eye by approximately 0.8 mm (p<0.05, Table 1). RNA from the sclera of three of these animals was chosen for gene array analysis. All three monkeys, RCZ6, REK7, and RQA7, showed statistically significant differences in axial length measurements of 1.1 mm, 1.3 mm, and 0.6 mm, respectively.
Expression array analysis
RNA isolation yielded 20-40 μg total RNA with an A260/280 ratio greater than 1.83 from sclera of both the aphakic and unmanipulated eye for monkeys RCZ6, REK7, and RQA7. Differential hybridization to the Atlas Human Cancer Gene Array membranes showed distinct hybridization patterns for the RNA from the sclera of the three monkeys for the operated versus the unmanipulated eye. Background values were subtracted, signals were normalized to G3PDH, and a threshold of two times background was used to select expressed genes. Individual signals were inspected and only those that showed specific hybridization were reported. This method potentially lowers the total number of reported genes but helps assure against false positive signals. There were 172 genes expressed for all three monkeys that gave distinct hybridization signals (Figure 1). For ease of presentation, transcripts represented on the arrays were classified into nine functional groups including: ECM, proteases, cell adhesion, cell cycle, apoptosis, transcription, cytoskeleton, cell surface receptors, and growth factors. Analysis of the autoradiograms demonstrated an increased expression of genes encoding transcripts for ECM, cell cycle, cell adhesion, cytoskeleton and cell surface receptors. There was little to no change in expression for genes encoding growth factors and transcription factors and a decreased expression of transcripts for proteases and apoptosis in the aphakic versus unmanipulated eyes.
The list of the genes which were expressed in at least 2 of the 3 monkeys in each of the nine functional groups in Table 2, Table 3, Table 4. Table 2 shows that for ECM, all transcripts were upregulated with those for collagen 3α1, decorin, collagen 8α1, collagen 6α3, SPARC, biglycan, and tenascin showing greater than twofold increases. In the protease category, the majority of transcripts were unchanged to slightly downregulated. Of the MMPs, MMP-7, MMP-11, MMP-14, and MMP-18 showed the greatest decrease while MMP-2 showed a slight increase. In general, TIMP-1 was unchanged, and TIMP-2, and TIMP-3 were decreased by twofold or greater. Transcripts for integrins were generally upregulated with the greatest increase in integrin α3, integrin α7B, and integrin αE. Of the integrin β transcripts expressed, integrin β8 increased, integrin β7 remained unchanged, and integrin β4 decreased slightly. Table 3 demonstrates that most growth factors expressed were unchanged. Notably, transcripts for basic FGF were expressed and slightly increased and transcripts for TGFβ2 were almost threefold increased, both known fibrogenic growth factors. Transcripts for growth factor receptors were generally unchanged with some variability up and down. One of the greatest changes was a greater than twofold increase in the FGF-4 receptor. Table 4 shows the transcripts associated with the cell cycle and demonstrates a general increase in transcript number. ERK1, ERK5, and ERK6 were some of the highest level transcripts and are known signal transducers for cell surface receptors. For apoptosis related transcripts, caspase-8, caspase-9, and caspase-10 were the only caspases detectable and all were downregulated in the aphakic eye.
The average expression change for the nine functional groups is shown in Figure 2. The greatest upregulation was seen in ECM, cell adhesion, cytoskeleton, and cell cycle, and the greatest downregulation was seen in apoptosis related transcripts. When the Bonferroni correction for multiple comparisons was applied, the only statistically significant changes in expression were for increased ECM, cell adhesion, cell cycle and decreased apoptosis. The data was further analyzed by K-means clustering with K=5 and expressed as sets 1 through 5 representing fold changes of greater than 2.00, 1.40 to 2.00, 1.00 to 1.40, 0.70 to 1.00, and less than 0.70, respectively (Figure 3). When all 172 expressed genes were considered, the distribution to the nine functional groups was fairly equal with a median value of 11% (Figure 3A). When grouped by expression values, ECM, cell cycle, and cell adhesion composed 80% of the genes showing greater than a 2.0 fold increase in the aphakic eye (Figure 3B). In the cluster with less than 2.0 fold and greater than 1.4 fold change, ECM, cell cycle, cell adhesion still composed 55% of the genes with cell receptors representing an additional 20% (Figure 3C). For transcripts showing less than 0.7 fold change, genes for apoptosis and protein turnover comprised 59% of that cluster (Figure 3D). When composition of the clusters was looked at a different way, 93% of ECM, 55% of cell cycle, 72% of cell adhesion, and 67% of cytoskeleton transcripts fell in Set 1 and 2 with greater than 1.4 fold increase in the aphakic eye (Figure 4). Additionally, 35% of protease, 55% of transcription, and 40% of apoptosis transcripts were represented in Set 4 remaining mostly unchanged and 22% of protease and 42% of apoptosis, represented in Set 5, showing decreased expression in the aphakic eye.
Discussion
In rhesus monkeys, the period of greatest ocular growth occurs in the first six months of life. In general, at birth, eyes are closely matched in size with a mean interocular difference of 0.12 mm [17]. Removal of the crystalline lens retards axial elongation in our primate model. We routinely observed greater than 0.4 mm axial length differences between the aphakic and the unmanipulated fellow eyes four to eight weeks postoperatively. The mechanisms underlying these changes have not been investigated in great detail. Our hypothesis predicts that removal of the crystalline lens alters the signals transmitted to the sclera from the lens directly or from the retina via the retinal pigment epithelium to the sclera leading to remodeling of the sclera, thus resulting in retardation of axial elongation. The degree of involvement of soluble factors produced by the lens versus the effect of defocus on these signals is still an area needing further investigation.
Overall, the genomes of humans and Rhesus macaques differ by 5-7.5% [18]. Because of evolutionary similarities between rhesus monkeys and humans, we decided to use Atlas Gene Arrays (human) to screen for changes in gene expression. Furthermore, extracellular matrix genes are fairly well conserved and, thus, lend themselves well to cross species analysis. Of the limited cDNA sequence for extracellular matrix genes in GenBank for Macaca mulatta represented on the Atlas gene array, sequence alignments with human sequence showed an average of 97% homology for the partial sequences available. We also chose a few genes that were detected on the array that had available Rhesus macaque cDNA sequence data and did alignments (blastn), which yielded on average 96% homology. Of course some gene sequences are more conserved than others. For example, genes on the array that were upregulated included collagen 8α1 and integrin β8, which shares 99% and 97% homology, respectively, with their human orthologs. Other genes positive from other functional groups on the array included chondroitin sulfate, 97%; TGFβ2, 96%; IGF-1, 98%; CDKN2D, 93%; CDK2, 95%; retinoic RXRB, 96%; and caspase-9, 93%. Genes on the array that were down regulated included MMP-11, MMP-14, and TIMP-4 which show 96, 97, and 95% homology, respectively.
There are limitations to cross-species gene array hybridization experiments. We tried to lessen these by designing our experiments such that three independent RNA samples obtained from different monkeys were analyzed in separate experiments. The data obtained were compared between experiments and only hybridization that was observed in at least two of the three experiments was reported. This would hopefully lower the number of false positives reported but could also underrepresent the total number of expressed genes. Also, reporting the data grouped into functional gene classifications allows the expression of multiple functionally related genes to be followed as compared to a single gene that may or may not be accurately represented.
With these potential limitations in mind, we observed increases in hybridization for ECM transcripts encoding proteoglycan protein backbones in the sclera of aphakic eyes. There is evidence in developmental models that the amount of proteoglycan and the degree of sulfonation can affect the pliability of the matrix involved [11]. Additionally, decorin and biglycan have been shown to bind TGFβ, increase its biological half-life, and help localize it to a specific site [13,14]. In this study we observed a close to 3 fold increase in expression of TGFβ2 in the aphakic eye. This may suggest a mechanism by which fibrogenic growth factors of the TGFβ family may be specifically localized in the sclera and lead to the deposition of new, and remodeling of old, ECM.
There was also a substantial increase in abundance of collagen, tenascin, and integrin transcripts in the sclera of aphakic eyes, as seen by gene array analysis. Sulfonation of the glycosaminoglycan side chains of proteoglycans can alter their mechanical properties such as tensile strength and pliability. The changes in mechanical properties of the scleral ECM are due in part to the ability of sulfonated proteoglycans to cross-link collagen fibrils and regulate water content in the sclera [11]. Insulin-like growth factor 1 (IGF-1) has been associated with an increased synthesis of sulfated proteoglycans, perhaps by upregulating gene transcription, translation or activation of the sulfotransferases [19]. Basic fibroblast growth factor (FGF-2) and TGFβ1 have also been associated with sulfated proteoglycan synthesis and may play a role in matrix changes seen during ocular growth [13,19]. Our analysis showed expression of both IGF-1 and FGF-2 in the sclera but without significant differences in the aphakic eye compared to the unmanipulated eye.
Form deprivation has been shown to alter the ECM content of the sclera in various animal models. Rada and colleagues demonstrated that form deprivation elicits myopia in a marmoset model and that synthesis of proteoglycans correlates inversely with vitreous chamber elongation [4]. Troilo et al. [5] reported that form deprivation myopia could be induced in mature marmosets and this ability to alter ocular growth persists past infancy and into maturity [5]. These companion studies examined proteoglycan and collagen content from isolated sclera. Similar studies were conducted in tree shrews looking at the changes in scleral composition for several ECM molecules. Induced myopia was associated with decreased levels of collagen type 1α1 [6] and both sulfated and unsulfated proteoglycans [7]. Additional studies with tree shrews showed that during development of myopia the transcipt levels for collagen type 1α1, MMP-3, and TIMP-1 decreased, decorin mRNA remained unchanged, and MMP-2 mRNA increased. Most interestingly, during the recovery period after the diffusion goggle was removed, mRNA levels for collagen type Iα1, decorin, MMP-3, and TIMP-1 increased to levels higher than control eyes while mRNA levels for MMP-2 decreased [8,9]. In this myopia recovery model, it was noted that the excessive elongation of the vitreous chamber was halted upon removal of the goggle. Further, after 2 to 4 days of recovery, the relative myopia and differences in vitreous chamber depths were progressively less than at the time of diffuser removal [8,9]. In our aphakia model, we observed a decrease in axial length that also correlated with an increase in expression of decorin, aggrecan, biglycan, type 3, 6, 8, 11, and 18 collagen, no change in TIMP-1 but a decrease in MMP-7, 11, and 14, not MMP-2, as compared to the unmanipulated companion eye. Taken together for mammalian models, these data suggest that during axial elongation, there is a catabolic process driving ECM remodeling characterized by a decrease in ECM production and an increase in ECM modifying enzymes. Conversely, a more metabolic profile that is characterized by an increase in ECM production and downregulation of ECM degrading enzymes is observed during aphakia-induced growth retardation and during recovery from induced myopia. Aside from changes in single genes, when we clustered the expressed transcripts by functional class a very interesting pattern emerged. We saw statistically significant upregulation of ECM, cell adhesion and to a lesser degree, cell cycle transcripts. We saw a trend towards downregulation of ECM degrading enzymes and proteases which was statistically significant without the Bonferroni correction. We also saw a concomitant downregulation of apoptosis transcripts. By comparing the expression of 14-33 genes per cluster and expression patterns between clusters, we could also minimize any bias from false positives inherent with single gene analysis.
All of these observations suggest that different stimuli applied to the growing and mature eye can modulate the remodeling of the scleral ECM. Of note in our experiments, one monkey, RHB-8, had a lensectomy in the right eye and a sham operation on the left eye. The growth and final intraocular differences between the eyes showed no difference from that seen in monkeys with an unmanipulated left eye (Table 1). Although a sham operation was done on only a single monkey, this data is suggestive that the physical trauma of the operation or manipulation itself and resulting inflammation is not contributing to the axial growth retardation. The stimulus that triggers this remodeling following the removal of the crystalline lens may be defocusing of light, loss of lens derived growth signals or most probably, a combination of the two mechanisms. The actual molecular mechanisms that underlie this process might originate from loss of soluble signals from the crystalline lens and/or in the retina and ultimately be manifested in the sclera as increased ECM production, resulting in reduced pliability and decreased axial elongation. Further studies examining gene expression in lens, retina, choroid, and retinal pigmented epithelium may help elucidate the signaling network that mediates changes in scleral ECM composition and remodeling and provide targets for control of ocular growth and ultimately, eye shape.
Acknowledgements
Supported by NIH grants EY08544, EY04864, and RR00165.
References
1. Lambert SR, Fernandes A, Drews-Botsch C, Tigges M. Pseudophakia
retards axial elongation in neonatal monkey eyes. Invest Ophthalmol Vis
Sci 1996; 37:451-8. ![]()
2. Boothe RG, Louden TM, Lambert SR. Acuity and contrast sensitivity
in monkeys after neonatal intraocular lens implantation with and without
part-time occlusion of the fellow eye. Invest Ophthalmol Vis Sci 1996;
37:1520-31. ![]()
3. Boothe RG, Louden T, Aiyer A, Izquierdo A, Drews C, Lambert SR.
Visual outcome after contact lens and intraocular lens correction of
neonatal monocular aphakia in monkeys. Invest Ophthalmol Vis Sci 2000;
41:110-9. ![]()
4. Rada JA, Nickla DL, Troilo D. Decreased proteoglycan synthesis
associated with form deprivation myopia in mature primate eyes. Invest
Ophthalmol Vis Sci 2000; 41:2050-8. ![]()
5. Troilo D, Nickla DL, Wildsoet CF. Form deprivation myopia in
mature common marmosets (Callithrix jacchus). Invest Ophthalmol Vis Sci
2000; 41:2043-9. ![]()
6. Norton TT, Miller EJ. Collagen and protein levels in sclera during normal development, induced myopia, and recovery in tree shrews. Invest Ophthamol Vis Sci 1995; 36:S760.
7. Norton TT, Rada JA, Clark EC. Proteoglycans in tree shrew sclera. Invest Ophthamol Vis Sci 1998; 39:S505.
8. Siegwart JT Jr, Norton TT. The time course of changes in mRNA
levels in tree shrew sclera during induced myopia and recovery. Invest
Ophthalmol Vis Sci 2002; 43:2067-75. ![]()
9. Siegwart JT Jr, Norton TT. Steady state mRNA levels in tree shrew
sclera with form-deprivation myopia and during recovery. Invest
Ophthalmol Vis Sci 2001; 42:1153-9. ![]()
10. Rada JA, Achen VR, Penugonda S, Schmidt RW, Mount BA.
Proteoglycan composition in the human sclera during growth and aging.
Invest Ophthalmol Vis Sci 2000; 41:1639-48. ![]()
11. Brown CT, Vural M, Johnson M, Trinkaus-Randall V. Age-related
changes of scleral hydration and sulfated glycosaminoglycans. Mech
Ageing Dev 1994; 77:97-107. ![]()
12. Muir H. Proteoglycans as organizers of the intercellular matrix.
Biochem Soc Trans 1983; 11:613-22. ![]()
13. Haase HR, Clarkson RW, Waters MJ, Bartold PM. Growth factor
modulation of mitogenic responses and proteoglycan synthesis by human
periodontal fibroblasts. J Cell Physiol 1998; 174:353-61.
![]()
14. Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR,
Border WA, Ruoslahti E. Interaction of the small interstitial
proteoglycans biglycan, decorin and fibromodulin with transforming
growth factor beta. Biochem J 1994; 302:527-34. ![]()
15. Wallman J, Turkel J, Trachtman J. Extreme myopia produced by
modest change in early visual experience. Science 1978; 201:1249-51.
![]()
16. Hoffer KJ. Ultrasound velocities for axial eye length
measurement. J Cataract Refract Surg 1994; 20:554-62. ![]()
17. Bradley DV, Fernandes A, Lynn M, Tigges M, Boothe RG.
Emmetropization in the rhesus monkey (Macaca mulatta): birth to young
adulthood. Invest Ophthalmol Vis Sci 1999; 40:214-29. ![]()
18. Stewart CB, Disotell TR. Primate evolution - in and out of
Africa. Curr Biol 1998; 8:R582-8. ![]()
19. Watanabe Y, Liu ZZ, Kumar A, Wallner EI, Kashihara N, Kanwar YS.
Influence of hypophysectomy on renal proteoglycans and their modulation
by insulin-like growth factor-I and its receptor. Endocrinology 1994;
134:358-70. ![]()