![]() Figure 3 of
Nickerson, Mol Vis 4:33, 1998.
Figure 3 of
Nickerson, Mol Vis 4:33, 1998.
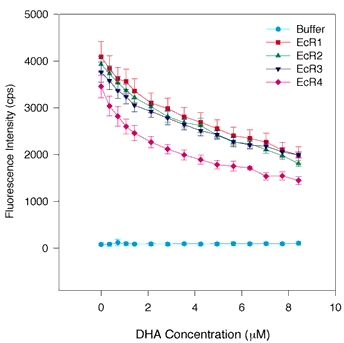
Figure 3. DHA quenching of tryptophan quenching of fluorescence in WT and individual repeats of IRBP
The same assay conditions were employed as in Figure 1. The symbols and colors are the same as in Figure 1. DHA appears to bind specifically to each of the four repeats. Qualitatively, there may be some nonspecific binding of DHA to the proteins indicated by the linear decrease in fluorescence of the proteins in the 4 to 8 µM range of DHA concentration. However, in the 0 to 3 µM range, the binding exhibits a hyperbolic character suggesting a saturable component to the quenching curve. This is borne out by curve fitting. The apparent Kds for DHA are somewhat smaller (Kd = 0.21 to 0.38 µM) than the Ki derived from competitive inhibition of retinol fluorescence enhancement (Ki = 0.78 µM) for bovine IRBP [18]. The error bars indicate the standard error of the mean.