![]() Figure 1 of
Nickerson, Mol Vis 4:33, 1998.
Figure 1 of
Nickerson, Mol Vis 4:33, 1998.
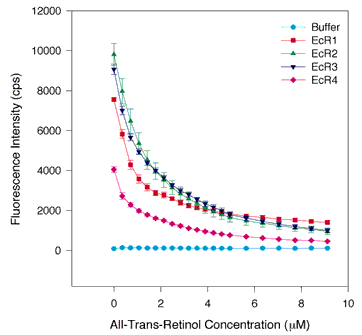
Figure 1. All-trans-retinol quenching of tryptophan fluorescence in WT and each individual repeat
One µM protein in 700 µl was placed in the cuvette and 0.5 to 1.0 µl aliquots of ligand in 100% ethanol were added. The total concentration of the ligand is shown on the abscissa, and the fluorescence intensity in photons counted per second (cps) is shown on the ordinate. Fluorescence was measured by excitation at 280 nm and emission at 336 nm. The raw data from all-trans-retinol titrations of the E. coli produced individual repeats are shown. The results from five or six independent assays are averaged. The error bars indicate the standard error of the mean. EcR1 is shown by the red squares, EcR2 is indicated by green triangles pointing up, EcR3 is denoted by blue triangles pointing down, and EcR4 is marked by magenta diamonds, and buffer is designated by aqua circles. The results show that tryptophans are quenched by all-trans-retinol in all four repeats, though not with identically shaped binding curves. The data imply that each repeat contains a saturable high affinity binding site for retinol.