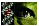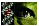
A Molecular Vision Research Article
Expression of Glial Markers in a Retinal
Precursor Cell Line
Gail M. Seigel,*
Aimee L. Mutchler, and
Eileen L. Imperato
Department of Neurobiology and Anatomy, University of Rochester School of
Medicine and Dentistry, 601 Elmwood Avenue Rochester, NY, 14642, (716) 273-4844.
*To whom correspondence should be addressed (E-mail:
GAIL_SEIGEL@urmc.rochester.edu).
ABSTRACT
Purpose: To determine glial characteristics of the retinal precursor cell line R28, which has previously been shown to express proteins immunoreactive with photoreceptor markers IRBP, S-Ag, recoverin, the ganglion cell marker 2G12, as well as the Muller cell marker RetG1.
Methods: R28, an immortalized retinal precursor cell line derived from P6 rat retinal tissue, was analyzed to determine expression of glial cell markers. R28 cells were analyzed both immunocytochemically and by western immunoblot for GFAP, S-100, and vimentin. These results were compared with the primary postnatal day 6 retina. Double fluorescence immunolabelling was used to identify R28 cells which simultaneously expressed vimentin and the photoreceptor marker IRBP (interphotoreceptor retinoid-binding protein).
Results: GFAP, S-100 and vimentin immunoreactive proteins were detected in R28 cells. Western blot analysis showed the GFAP immunoreactive band to migrate at a slightly higher apparent molecular weight for R28 than for P6 retina, and demonstrated a less fibrillary staining pattern than P6 retina, but appeared to be present to some degree in all R28 cells. Variations in molecular weight were seen for S-100, although the nuclear staining pattern was the same for both the R28 cell line and P6 retina. S-100 immunoreactivity was seen in approximately 50% of the R28 cell population. Vimentin was expressed by virtually all R28 cells, and to a greater degree than that seen in P6 retina (both in cell number and intensity). Double labelling studies revealed R28 cells which expressed both vimentin and IRBP simultaneously.
Conclusions: There is a very strong glial component to the R28 retinal precursor cell line, as evidenced by the expression of proteins immunoreactive to GFAP, S-100 and vimentin. However, even the most strongly immunoreactive marker vimentin was compatible with co-expression of the photoreceptor marker IRBP as evidence of the "multi-phenotypic" nature of the precursor-like R28 cells. Ongoing studies will assess the differentiation potential of R28 cells and applicability in future studies of retinal cell differentiation and gene expression.
INTRODUCTION
The R28 retinal precursor cell line was developed as an alternative to transformed, tumorigenic retinal cell lines (12). The 12S portion of the E1A gene was used for immortalization, as it contained the immortalizing, but not the transforming functions of the gene (16). The resulting retinal cell line R28 was shown to express several markers characteristic of photoreceptor cells, as well as the ganglion marker 2G12 (18). The possibility of Muller cell marker expression was evidenced in that sudy by expression of RetG1. In this study, we sought to further characterize the glial cell component of this cell line to better understand the phenotypic state of these cells as we seek evidence of their multipotentiality.
Glial cell markers have been useful histological tools in studies of retinal development and cellular origin. The intermediate filament vimentin is expressed ubiquitously in Muller cells of many mammalian species (6), while GFAP appears to be upregulated in reactive states (1), and developmentally regulated in retinal glia. (10) S-100 is an acidic protein widely used as a glial marker, although there are indications that it may also be present in ganglion cells. (3) In this study, we studied the expression of GFAP, S-100 and vimentin in the R28 retinal precursor cell line and compared it with the in vitro P6 rat retinal tissue of origin. In this study,in vitro cultures were compared for the purpose of consistency, as well as comparability of the R28 cell line to P6 rat retina grown in vitro . Our results indicate that subpopulations of both the cultured P6 rat retina and the R28 cell line express all of these markers in vitro , to varying degrees, but differ in some ways with regard to the nature and extent of glial marker expression.
MATERIALS AND METHODS
Cell Culture
Primary retinal tissue was obtained from postnatal day 6 Sprague-Dawley rats (Charles River, NJ). Animals were used in accordance with the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research. Under a stereo microscope, neuralretinae were carefully dissected free from pigmented epithelium and optic nerve and placed in calcium-magnesium-free buffer (CMF) with 50 µg/ml gentamicin. Tissues were plated as explants in organ culture dishes in DMEM supplemented with 10% fetal bovine serum, 1X MEM non-essential amino acids (GIBCO), 1X MEM vitamins (GIBCO), 1% Tryptose phosphate broth, and 50 µg/ml gentamicin.
Establishment of the cell line R28, and the parental culture designated E1A-NR.3 is described previously (12, 18). Briefly, a Psi2 12S E1A replication-defective retroviral vector was used to immortalize P6 rat retinal tissue. Infected cells underwent two weeks of selection on the basis of neomycin resistance. The R28 cell line was derived after three rounds of cloning by limiting dilution to ensure that the final clonal population originated from one single cell.
Immunocytochemistry
Cells were fixed for 10 minutes at room temperature in 2% paraformaldehyde and permeabilized in 0.25% Triton X-100 for five minutes. After a rinse in phosphate-buffered saline (PBS), cells were incubated for one hour with a primary antibody. After rinsing 3 x 5 minutes in PBS, cells were incubated with biotinylated goat anti-rabbit or anti-mouse immunoglobulin (Vector Laboratories, Burlingame, CA) for 60 minutes. Cells were equilibrated in Tris-buffered saline (50 mM Tris-HCl, pH 7.6, 0.9% NaCl) and incubated for 20 minutes with horseradish peroxidase-conjugated avidin (Elite kit, Vector Laboratories). The cells were rinsed in 0.05M Tris and developed with a diaminobenzidine (DAB) kit (Pierce) and the brown/black reaction product was visualized by light microscopy. Negative controls consisted of incubations in control serum without primary antibody, and did not generate reaction product. Cells of neuronal morphology in the primary retinal cutures were used as internal negative controls, and can be seen in some figures.
For immunostaining, primary antibodies included: rabbit anti-GFAP (Dako, Carpinteria, CA) 1: 5,000; rabbit anti-S-100 (Dako) 1:100; vimentin mouse monoclonal (Boehringer Mannheim) 1:3; rabbit anti-interphotoreceptor retinoid binding protein (anti-IRBP) (courtesy of Dr. B. Wiggert) (17) 1: 750.
Double immunofluorescent staining utilized vimentin and IRBP primary antibodies in combination at the aforementioned concentrations. Fluorescent secondary antibodies were used at a 1: 300 dilution in combination, as well. A rhodamine-linked goat anti-mouse Ig (Zymed, So. San Francisco, CA) was used to detect vimentin primary antibody, while an FITC-linked goat anti-rabbit antibody (Zymed) was used to detect IRBP primary antibody. Controls consisted of single-labelled cells to assure that no "bleed-through" of fluorescence was observed with opposing filters. Negative controls consisting of cells incubated in normal goat serum and secondary antibody remained non-fluorescent.
Western immunoblot
Analysis of E1A expression was carried out by means of Western immunoblot analysis. (9, 11) Cells were removed from culture with 0.125% trypsin-EDTA, resuspended in 10% sodium dodecyl sulfate, and boiled for ten minutes. Clumps were broken up by passing the sample through a 23 gauge needle. Nucleic acids and debris were pelleted by a 10 minute, 10,000 rpm centrifugation. The supernatant was saved, transfered to a fresh tube, and stored at -70oC until use.
To ensure that equal aliquots of sample were removed for analysis, cell lysates were analyzed for protein content by the BCA method (Pierce Biochemicals). Samples containing 25µg protein were then reduced with 100mM dithiothreitol and loaded into the wells of a 5% stacking/10% running polyacrylamide gel. Electrophoresis was carried out at 30 mAmps for one hour and then the proteins were transferred overnight to nitrocellulose by electroblotting at 30 mAmps in 25mM Tris-Cl, 192 mM glycine, and 20% methanol.
Proteins on nitrocellulose were detected following a 1 hour blocking step in 10% Gold Cow non-fat dry milk. (5) The blot was then incubated with primary antibodies, as follows: GFAP 1: 1,000; S-100 1: 100; vimentin 1: 100. After a 1 hour incubation the blot was washed extensively in TBS-Tween (25mM Tris-Cl, 0.2 mM NaCl, 0.1% Tween - 20). The washed blot was incubated in peroxidase-linked sheep anti-mouse immunoglobulin (Amersham) for 1 hour, and the wash steps repeated. Signals were generated on autoradiographic film by enhanced chemiluminescence (Amersham) (15).
RESULTS
 Negative Controls
Negative Controls
In order to assess background levels of cell staining, negative controls incubated in normal goat serum as the primary non-specific antibody are shown in Figure 1 for both R28 and the P6 retina. For all cell staining, either goat anti-rabbit (GFAP and S-100) or goat anti-mouse (vimentin) secondary antibodies were used. Both sets of these negative controls showed no significant immunoreactivity.
 GFAP
GFAP
Figure 2 illustrates P6 retina and R28 immunoreactivity to GFAP, both immunocytochemically, and by western immunoblot. Figure 2a shows the classic, fibrillary staining pattern of GFAP as seen in the large, flat cells of the P6 retina. This fibrillar pattern is not evident in R28 cells (Figure 2b), but is seen as a generalized immunoreactivity of varying, but lower intensity within virtually all cells. Figure 2c indicates a slight difference in apparent molecular weight and intensity of the GFAP bands between P6 retina and R28 cell lysates. The primary cell lysate (lane 1) shows a more intense immunoreactive band, while the R28 cell lysate exhibits a less intense band of approximately 3 kD higher molecular weight.
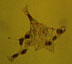 S-100
S-100
Figure 3 displays P6 retina and R28 immunoreactivity to S-100, as analyzed immunocytochemically, and by western immunoblot. Figure 3a shows the nuclear staining pattern of S-100 as seen in the large, flat cells of the P6 retina, which is also seen in R28 with more intensity (Figure 3b). Cell counts indicate approximately 50% of the cells in the R28 population exhibit S-100 immunoreactivity. Figure 3c indicates the difference in apparent molecular weights and intensities of the S-100 immunoreactive bands between P6 retina and R28 cell lysates. The lower, standard 23kD S-100 band is present in the primary cell lysate (lane 1, arrowhead), but not the R28 cell lysate (lane 2). Another S100 immunoreactive doublet of approximately 50 kD is present in the primary cell lysate (lane 1), with a slightly heavier doublet for the R28 cell lysate (lane 2).
 Vimentin
Vimentin
Figure 4 represents P6 retina and R28 immunoreactivity to vimentin, as seen immunocytochemically, and by western immunoblot. Figure 4a shows the intermediate filament staining pattern characteristic of vimentin as seen in the large, flat cells of P6 retina, which is also observed in R28, but with more intensity and in virtually all cells (Figure 4b). In Figure 4c, the molecular weight of vimentin appears identical between P6 retina and R28 at 51 kD, but enhanced intensity of the vimentin band can be visualized for R28 (lane 2).
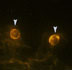 Double-labelling studies
Double-labelling studies
In order to address the question of multiple phenotypes in the R28 cell line, double-labelling studies were carried out to determine if more than one type of marker could be expressed in a single cell. For technical reasons, two antibodies were used which required contrasting secondary antibodies, so that potential cross-reactivity could be avoided. The two markers selected were vimentin, due to its strong immunoreactivity in virtually all cells, and IRBP, because of its high level of expression in many of these cells. Figure 5 shows two panels taken of the same field. In cells marked with arrowheads, both vimentin and IRBP staining can be seen simultaneously in a single cell. In addition, there were cells immunoreactive for one marker and not the other. Vimentin positive cells without IRBP immunoreactivity can be seen in Figure 5a, but not Figure 5b.
DISCUSSION
R28 was previously shown to display characteristics consistent with at least photoreceptor, ganglion, and even Muller cell phenotypes (18). In light of the present findings, it appears that R28 cells are at a pre-glial/neuronal commitment stage. Developmental studies indicate that similar neuroepithelial cells act as pluripotential precursors of mature neurons and glia, as multiple phenotypes arise from a single labelled precursor (13). With appropriate treatments of immortalized precursor cells, it may be possible to obtain large numbers of mature, homogeneous cell populations not possible with primary cell culture systems. The use of specific neurotrophic/gliotrophic agents to promote commitment of R28 progenitor cells is underway.
Despite the clonal cell origin of the R28 cell line, there were variations in the nature and extent of glial marker expression within the cell population. This suggests that there are individual differences between R28 cells grown under identical conditions. Thus, these precursor cells may encompass a broad range of phenotypic states and levels of maturity, as evidenced ultimately by co-expression of vimentin and IRBP within specific cells. Heterogeneity within chick retinal neuroepithelial cell populations suggests that differentiation of these progenitor cells may proceed through intermediate stages as cell fate becomes progressively restricted (4). Since cell culture conditions were unchanging in that study as well as in ours, it suggests an intrinsic program, rather than a solely environmental influence on cell heterogeneity.
There were also some interesting differences between R28 and the P6 retina from which it was derived. For R28, GFAP and vimentin appeared to be ubiquitous, while S-100 was clearly present in only about 50% of the population. The variations between R28 and P6 retina in apparent molecular weight (for GFAP and S-100) and staining pattern (for GFAP) may reflect the effects of the immortalization process. As with S-100 (14), these proteins may be a family of immunoreactive proteins with differing molecular weights dependent upon the proliferative or differentiated state of the cell. Molecular weight and staining pattern differences may reflect changes in post-translational modifications, as manifested by 12S E1A expression and induction of proliferation. Further observations at the electron microscopic level may allow for more detailed ultrastructural observations of the cytoskeletal/intermediate filament composition of R28 cells, as compared to the P6 retina. In addition, the use of anti-mitotic agents may determine how much of these differences are dependent upon the proliferative state of the immortalized cells.
In this study, we have compared the R28 cell line with cultured P6 retinal tissue. This was primarily an in vitro study, however we must take into account potential differences in glial marker expression of P6 rat retinal cells in vivo . As mentioned previously, GFAP appears to be upregulated in reactive states (1), including cell culture, and may not be as prominent in vivo . Transplantation studies are ongoing which will address the effects of the in vivo retinal milieu on glial and other retinal marker expression in 12S E1A immortalized retinal cells, which can then be compared with the normal retina in situ. As for in vitro studies, the R28 cell line appears to have many applications.
The potential applications for the clonal R28 cell line are potentially even more powerful than the original, uncloned mixed parental cell culture E1A-NR.3. E1A-NR.3 cells have been used as a system for the study of IRBP promoter gene regulation, and found to be comparable to normal retina (2). In addition, the parental cells have been useful for studies of antibody effects on retinal cells (7, 18) and drug therapy studies (8, 18). As we further assess the differentiation potential of R28 cells as a population, we hope to be able to address important questions regarding cell lineage and mechanisms of differentiation.
Acknowledgements
The authors thank Linda Liu, and Lois Chu for technical support. We also thank Dr. Roger Cone (E1A retroviral vector) and Dr. Barbara Wiggert for the IRBP antibody. Supported in part by NIH grant EY10676 and The Rochester Eye and Human Parts Bank (G.M.S.).
REFERENCES
1. Bignami, A., and D. Dahl, The radial glia of the Muller in the rat retina and their response to injury. An immunofluorescence study with antibodies to the glial fibrillary acidic (GFA) protein. Exp. Eye Res. 28 (1979) 63-79. 
2. Fei, Y., G. M. Seigel, and G. I. Liou, The use of an E1A-immortalized retinal culture to study the regulation of the interphotoreceptor retinoid-binding protein gene. Investigative Ophthalmol. & Vis. Sci. 37 (1996) S337.
3. He, W., and H. Inomata, Dual immunological property of S-100 protein in normal eyes and eye with retinoblastoma: A histopathological and immunohistochemical study of 88 cases. Ophthalmologica. 206 (1993) 133-138. 
4. Hernandez-Sanchez, C., J. Frade, and E. de la Rosa, Heterogeneity among neuroepithelial cells in the chick retina revealed by immunostaining with monoclonal antibody P1. Eur. J. Neurosci. 6 (1994) 105-114. 
5. Johnson, D., J. Gautsch, J. Sportsman, and J. Elder, An improved technique utilizing nonfat dry milk for analysis of proteins and nucleic acids transferred to nitrocellulose. Gene Anal. Tech. 1 (1984) 3-8.
6. Kivela, T., A. Tarkkanen, and I. Virtanen, Intermediate filaments in the human retina and retinoblastoma. Inv. Ophthalmol. & Vis. Sci. 27 (1985) 1075-1084.
7. Machnicki, M., G. M. Seigel, and G. Adamus, Evidence for the pathogenic effect of anti-recoverin antibodies in cancer-associated retinopathy. Invest. Ophthalmol. & Vis. Sci. 37 (1996) S535. Abstract
8. Ragaiey, T., J. X. Ma, G. M. Seigel, W. J. Jiang, and W. C. Stewart, L-Deprenyl increases the survival of retinal cell line E1A-NR.3 dying by apoptosis. Invest. Ophthalmol. & Vis. Sci. 37 (1996) S830. Abstract
9. Sambrook, J., E. Fritsch, and T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual, 2 ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York.
10. Sarthy, P., M. Fu, and J. Huang, Developmental expression of the glial fibrillary acidic protein (GFAP) gene in th mouse retina. Cell. Molec. Neurobiol. 11 (1991) 623-637. 
11. Seigel, G., and M. F. D. Notter, Lectin-induced differentiation of transformed neuroretinal cells in vitro . Exp. Cell Res. 199 (1992) 240-247. 
12. Seigel, G. M., Establishment of an E1A-immortalized retinal cell line. In vitro Cellular and Developmental Biology. 32 (1996) 66-68. 
13. Turner, D., and C. Cepko, A common progenitor for neurons and glia persists in the rat retina late in development. Nature. 4 (1987) 833-845.
14. Vincendon, G., J. Zanetta, and G. Combos, The heterogeneity of the S-100 protein fraction. Adv. Exp. Med. Biol. 32 (1970) 9-15.
15. Whitehead, T., G. Thorpe, T. Carter, C. Groucutt, and L. Kricka, Enhanced luminescence procedure for sensitive determination of peroxidase-labelled conjugates in immunoassay. Nature(London). 305 (1983) 158-159.
16. Whyte, P., H. Ruley, and E. Harlow, Two regions of the adenovirus early region 1A proteins are required for transformation. J. Virol. 62 (1988) 257-265. 
17. Wiggert, B., L. Lee, M. Rodrigues, H. Hess, T. Redmond, and G. Chader, Immunochemical distribution of interphotoreceptor retinoid binding protein in selected species. Inv Ophthalmol and Vis. Sci. 27 (1986) 1041-1049.
18. Imperato, E. L., A. L. Mutchler, G. Adamus, C. Barnstable, and G. M. Seigel, Establishment and characterization of immortalized retinal precursor cell lines: R28 and R57. Unpublished data.
 Return to Molecular Vision homepage
Return to Molecular Vision homepage
Received 15 March 1996 | Revised 10 April 1996 | Accepted 22 April 1996 | Uploaded 24 April 1996
Referencing Note: This article may be referenced as: Mol. Vis. 2:2, 1996.
Alternatively, this article may be referenced by its unique URL:
http://www.emory.edu/molvis/v2/seigel
© 1996 Molecular Vision
 Negative Controls
Negative Controls