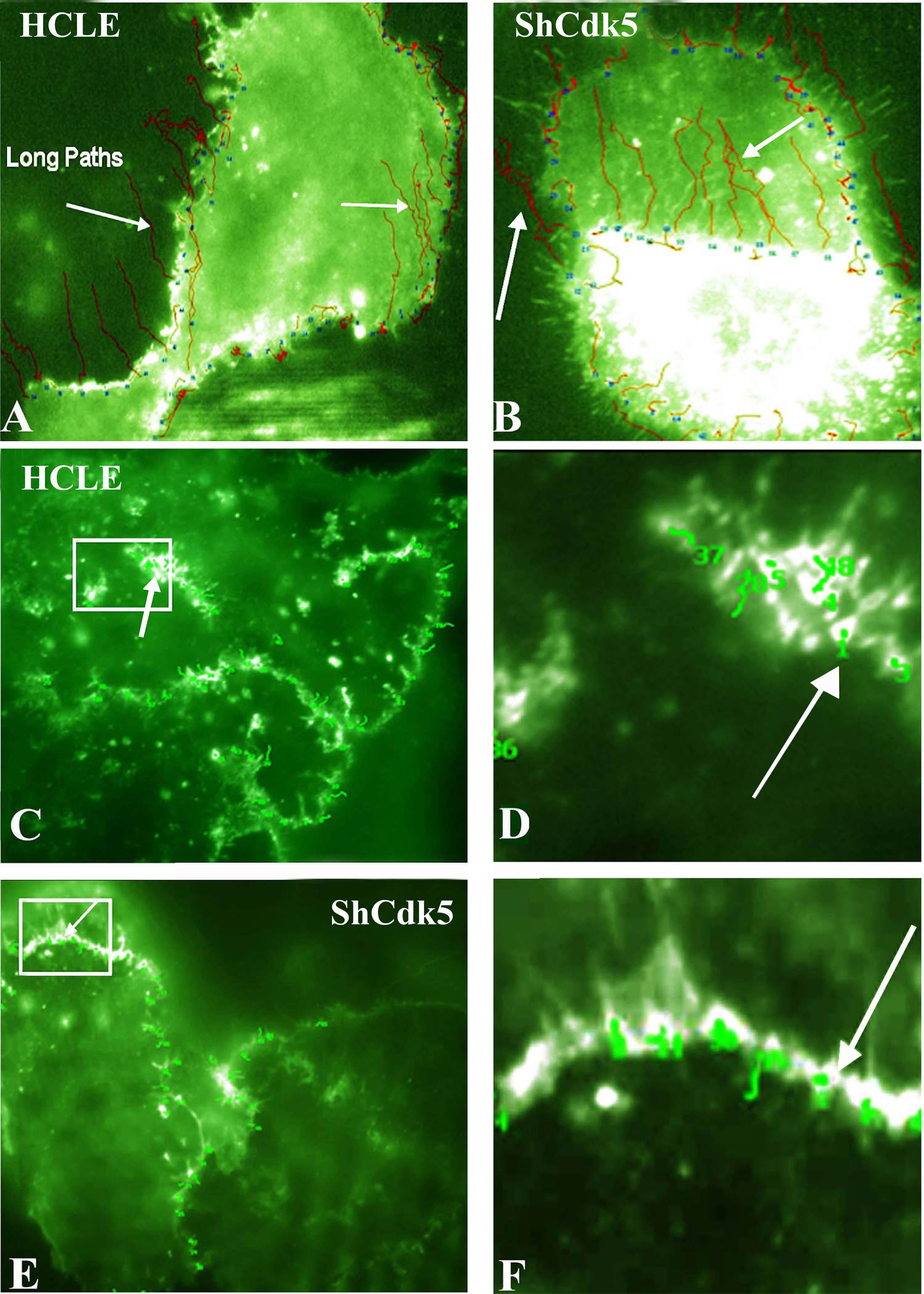
Figure 5. Total internal reflection fluorescence analysis for the E-cadherin internalization pathway in the human corneal limbal epithelial
and ShCDK5 cells. Green fluorescent protein (GFP)-E-cadherin transfected (
A–
B) epithelial cells with and without olomoucine and ShCDK5 cultures were analyzed for E-cadherin particle movement for distance
and tortuosity from the cell–cell borders at a constant time (
A). The tortuosity or particle tracking of the E-cadherin-containing vesicles was recorded. The E-cadherin vesicles moved in
long paths (arrows) from the cell borders to the interior, and such long paths were significantly greater in the ShCDK5 suggestive
of internalization than the control human corneal limbal epithelial (HCLE) cells. Total internal reflection fluorescence (TIRF)
analysis for the p120 (
C–
H) shows internalization pathway in the HCLE and ShCDK5. A GFP-p120 clone was transfected into the HCLE and ShCDK5 cells, and
the vesicle tracking (arrows) for the tortuosity was measured. P120 vesicles remained in the cell–cell borders, and no long
paths were internalized. In the HCLE and ShCDK5 cells, the p120 tortuosity paths were restricted within the cell–cell borders.
Panel inserts in the HCLE cells transfected with p120 (
C) and ShCdk5 transfected with p120 (
E) are enlarged (zoom to a factor of 2) and shown in
D and
F, respectively, along with the tortuosity path for each junctional vesicle analyzed. The distance and tortuosity of paths
by the E-cadherin- and p120-containing particles (n>100) were tracked at a constant time for all the particles analyzed using
TIRF microscopy are described in
Table 1. The E-cadherin-containing particles in the ShCdk5 cells moved fast, and these long, straight paths were internalized (
B). The p120-containing particles in the ShCdk5 cells moved slowly and stayed near the cell–cell boundary (
C–F). In the absence of CDK5, the E-cadherin and p120 junctional complex dissociate, promoting internalization and endocytosis
of E-cadherin. Cdk5 stabilizes the junction by preventing E-cadherin-containing vesicles from being endocytosed. 100X images.
 Figure 5 of
Arpitha, Mol Vis 2013; 19:319-332.
Figure 5 of
Arpitha, Mol Vis 2013; 19:319-332.  Figure 5 of
Arpitha, Mol Vis 2013; 19:319-332.
Figure 5 of
Arpitha, Mol Vis 2013; 19:319-332.