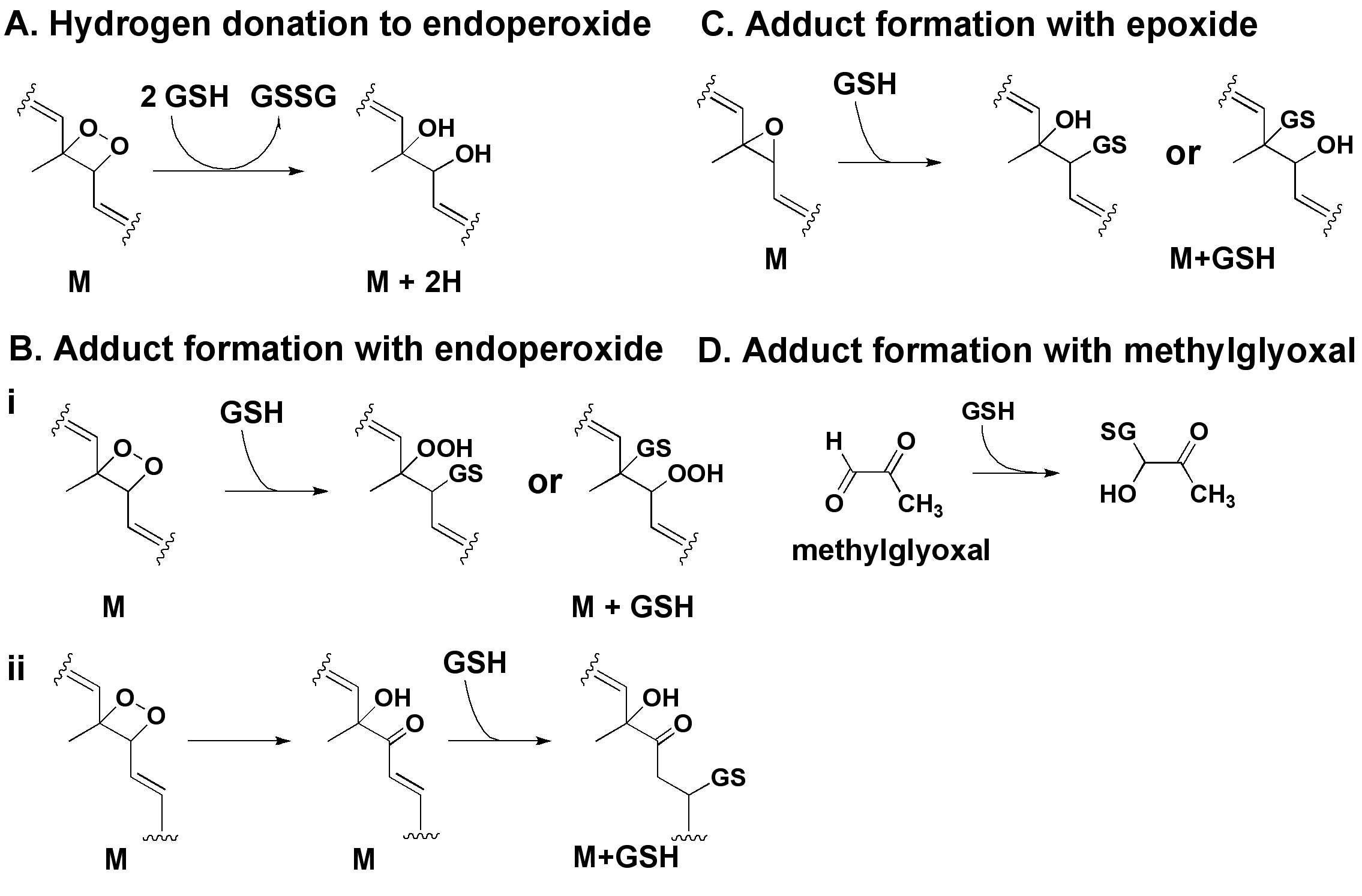
Figure 7. Proposed mechanisms for
glutathione (GSH) interaction with photooxoA2E and methylglyoxal, an
A2E photodegradation product.
A: GSH would transfer two
hydrogens from two GSH molecules to an endoperoxide on A2E, resulting
in the
m/z+2 pattern associated with photooxidized forms of A2E
in the
m/z 640–736 region of
Figure 3B-D [
33].
B: GSH
adduct formation with an endoperoxide on A2E would occur via
nucleophilic attack and ring opening [
34,
35].
Simple
addition of GSH at the site of an endoperoxide would involve the
formation of an unstable hydroperoxide (OOH) moiety (
B, i) and
for example would account for
m/z 947 in
Figure 4B,
insert. Alternatively, GSH conjugation could involve attack of the
endoperoxide bridge (O-O) by the GSH thiolate followed by carbonyl
formation and GS insertion (
B, ii); this mechanism would account
for
m/z 931 in
Figure 4B, insert [
33,
36,
37].
C: Adduct
formation with an epoxide would be expected to occur [
35];
however, the
appropriate product (
m/z 915) was not detected.
D: GSH
can react with methylglyoxal (MG) released upon A2E photodegradation to
form an MG-GSH hemi-thioacetal [
38];
this adduct accounts for
m/z 380
and 402 in
Figure
6F.
 Figure 7 of Yoon, Mol Vis 2011; 17:1839-1849.
Figure 7 of Yoon, Mol Vis 2011; 17:1839-1849.  Figure 7 of Yoon, Mol Vis 2011; 17:1839-1849.
Figure 7 of Yoon, Mol Vis 2011; 17:1839-1849.