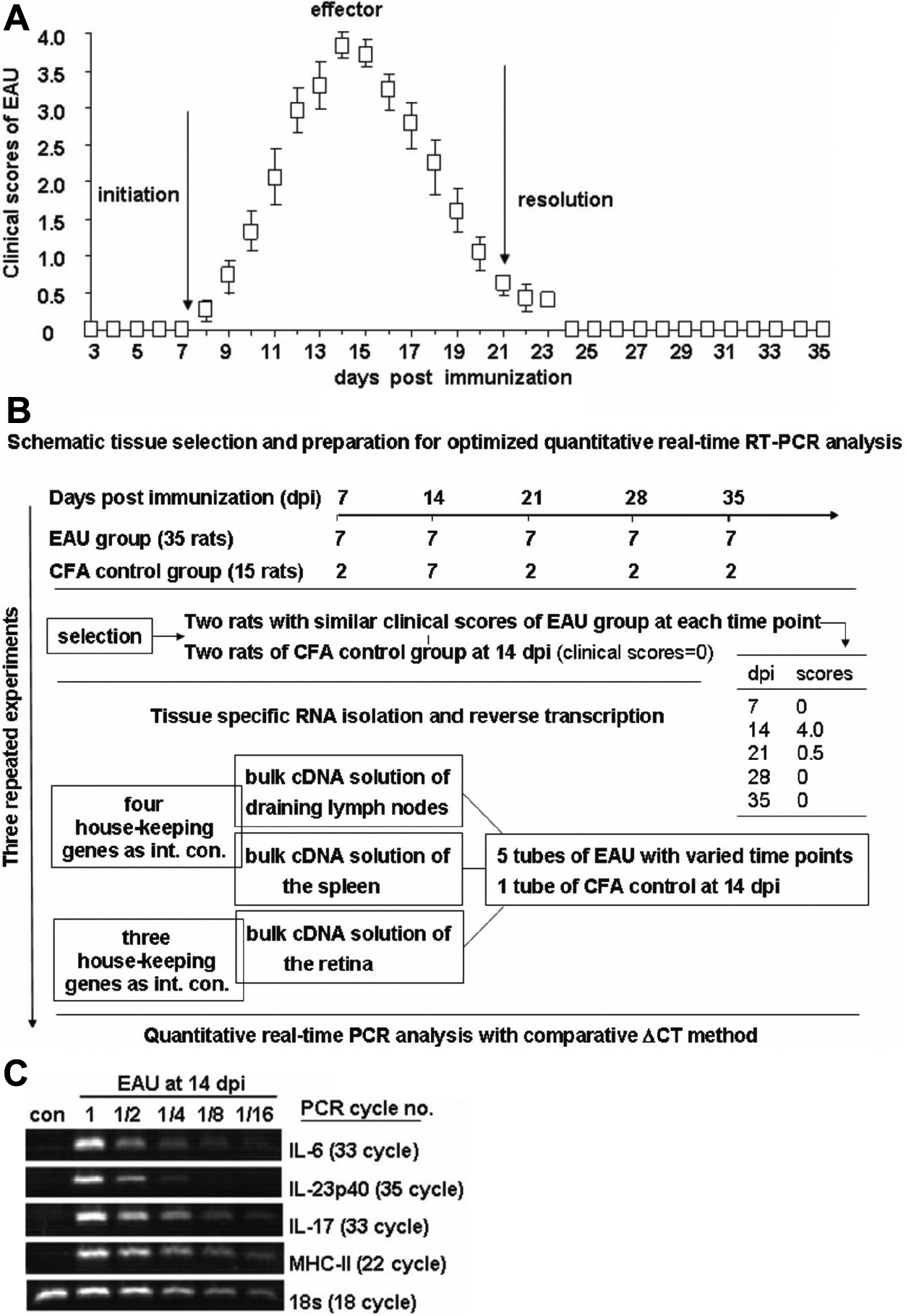
Figure 1. Clinical score of the monophasic EAU and methodology of real-time PCR analysis.
A: Clinical scores of the monophasic experimental autoimmune uveitis (EAU). Disease severity was observed daily by slit-lamp
microscopy and graded as described in the Methods section. Based on the clinical course, monophasic EAU was divided into three
stages: an initiation phase from the day of immunization to 7 dpi; an effector phase beginning from 7 to 21 dpi, with the
peak inflammation obtained at 14 dpi; and a phase of resolution starting from 21 dpi. Data are represented as the mean±standard
deviation.
B: Schematic tissue selection and preparation for optimized quantitative real-time RT–PCR analysis was shown.
C: Validation of the results of quantitative real-time PCR by traditional PCR. Traditional PCR using the series diluted cDNA
of EAU retina at 14 dpi and undiluted cDNA from the CFA control was performed, and the relative expression of the selected
factors was compared with the results from quantitative real-time PCR. In line with the most significant 5,202 fold upregulation
at EAU 14 dpi on MHC-II expression by real-time PCR analysis (
Table 3), its relative expression was the most abundant as compared to that of other individually examined factors, e.g.,
IL-17,
IL-23p40, and
IL-6, by traditional PCR measurement. A visible PCR band on electrophoresis by using as little as 1/16 dilution of the original
cDNA was observed in the series PCR for the expression of
MHC-II with the lowest 22 PCR cycles compared to that of other sets of relevant PCR data, e.g.,
IL-17 with 33 cycles. Data represent three repeated experiments.
 Figure 1 of
Jia, Mol Vis 2011; 17:1493-1507.
Figure 1 of
Jia, Mol Vis 2011; 17:1493-1507.  Figure 1 of
Jia, Mol Vis 2011; 17:1493-1507.
Figure 1 of
Jia, Mol Vis 2011; 17:1493-1507.