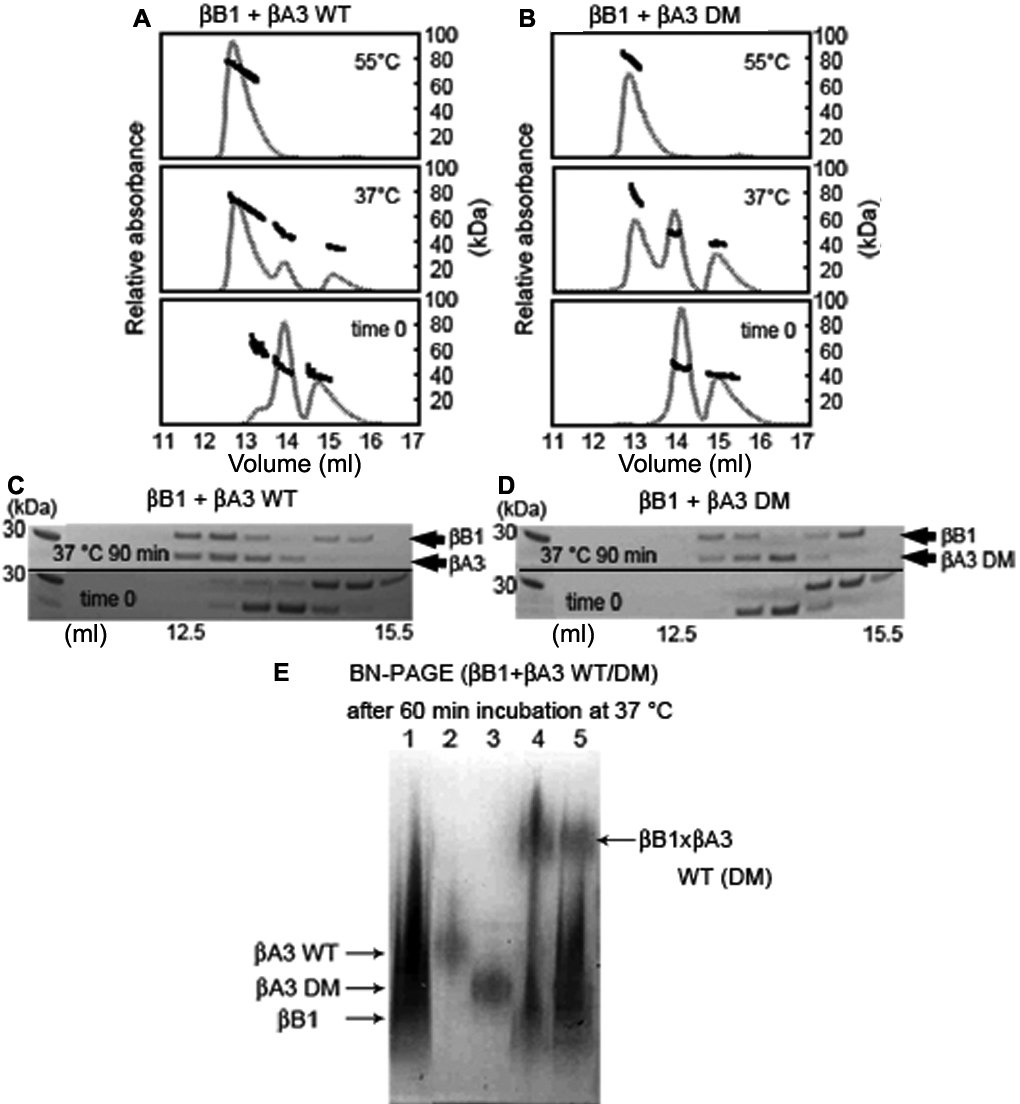
Figure 3. Hetero-oligomerization of βB1 with βA3. Similar size oligomers were obtained when βB1 was mixed with either βA3 WT (
A) or βA3 DM (
B). Mixtures were immediately subjected to SEC-MALS (bottom panel) or incubated at 37 °C (middle panel) or 55 °C (top panel)
for 90 min as in
Figure 2. Note the decreased amount of the βB1:βA3 DM compared to the βB1:βA3 WT complex. Eluting fractions of βB1 mixed with either
βA3 WT (
C) or βA3 DM (
D) were collected and subjected to SDS-PAGE. Both subunits, βB1 and βA3 WT or DM, were present in the complex. Hetero-oligomer
formation was confirmed by subjecting mixtures of βB1 with βA3 WT or βA3 DM incubated at 37 °C for 60 min to Blue-Native-PAGE
(
E). Proteins were βB1 (lane 1), βA3 WT (lane 2), βA3 DM (lane 3), βB1:βA3 WT (lane 4), and βB1:βA3 DM (lane 5).
 Figure 3 of
Takata, Mol Vis 2009; 15:241-249.
Figure 3 of
Takata, Mol Vis 2009; 15:241-249.  Figure 3 of
Takata, Mol Vis 2009; 15:241-249.
Figure 3 of
Takata, Mol Vis 2009; 15:241-249.