Figure 2. Homo-oligomerization of
β-crystallins. Purified crystallins were incubated for 90 min at either
37 °C or 55 °C and then subjected to SEC-MALS. Eluting proteins were
detected by UV absorption at 280 nm (grey line). Molar masses were
determined across the peak (solid squares). From molar masses, βB1 was
a mixture of monomer-dimers (A); βB2 was predominantly a dimer
with less of an earlier eluting peak (B); βA3 WT was a dimer (C);
and βA3 DM was a dimer at 37 °C, but had precipitated at 55 °C (D).
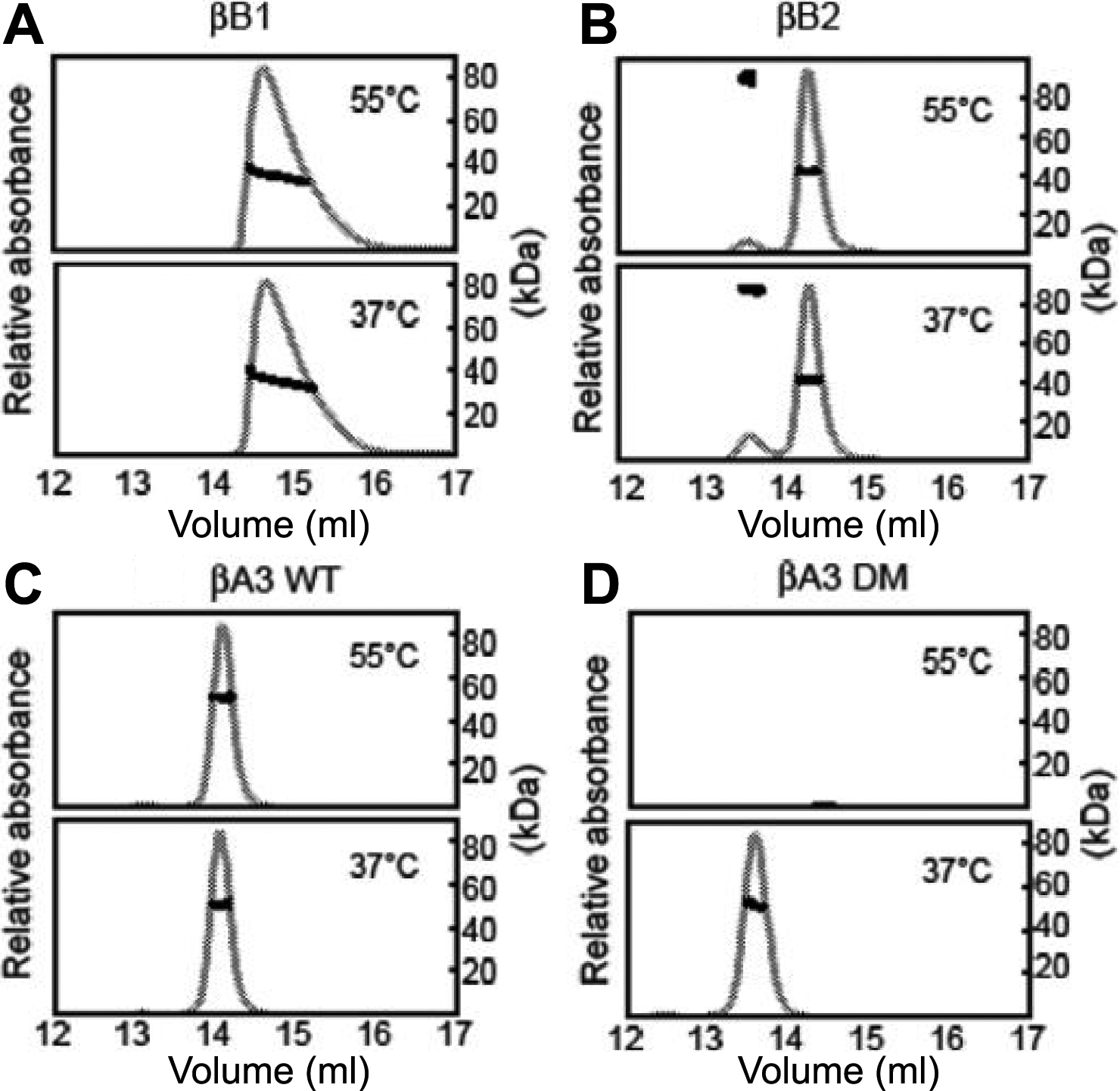
 Figure 2 of Takata, Mol Vis 2009; 15:241-249.
Figure 2 of Takata, Mol Vis 2009; 15:241-249.  Figure 2 of Takata, Mol Vis 2009; 15:241-249.
Figure 2 of Takata, Mol Vis 2009; 15:241-249. 