![]() Figure 4 of
Warburton, Mol Vis 2005;
11:1122-1134.
Figure 4 of
Warburton, Mol Vis 2005;
11:1122-1134.
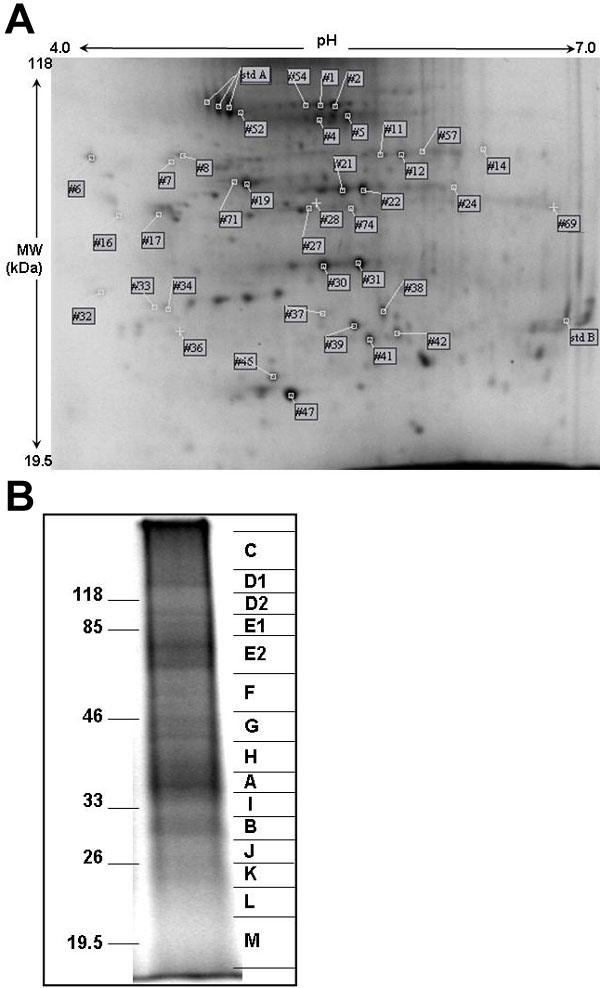
Figure 4. Electrophoresis of RLF proteins
A: 2D electrophoresis gel from 300 μg of RLF protein. This gel is representative of gels from four separate RLF preparations. Spots found in at least three of the four gels are indicated by their identifying spot number (Table 1). Spots lacking a label were unique to this particular preparation. Spots from standard proteins used to align gels are indicated as "std A" and "std B". B: Representative lane of SDS-PAGE of 50 μg of RLF protein. Mobilities of molecular weight markers are indicated to the left. On the right, gel slices taken for subsequent in-gel digestion are shown. The 1D gel shows relatively few well-resolved bands with much diffuse protein staining; thus 6 fold more total protein is needed in the 2D gel in order to observe a surprisingly small number of well-focused protein spots. Taken together, these observations suggest that the proteins of RLF are notably heterogeneous.