![]() Figure 10 of
Fujii, Mol Vis 2003;
9:315-322.
Figure 10 of
Fujii, Mol Vis 2003;
9:315-322.
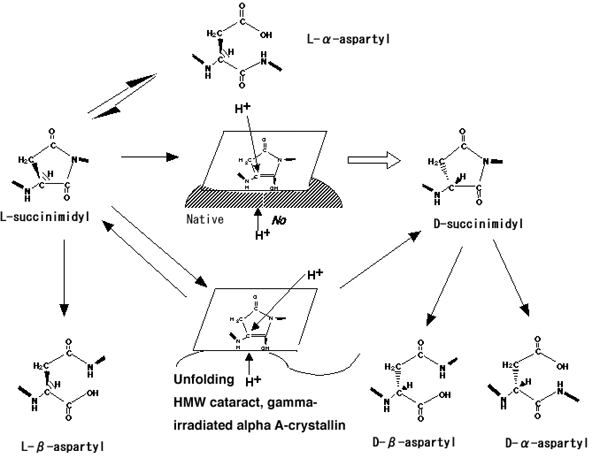
Figure 10. Reaction pathways of inversion and isomerization of Asp residues in protein
Reaction pathways for spontaneous inversion and isomerization of aspartyl residues in protein via a succinimide intermediate. A sterically hindering structure composed of the native higher order structure of αA-crystallin may be present on the lower side of intermediate [I] (shaded part), resulting in the protonation of intermediate [I] from the upper side of the plane, causing the configuration to be inverted to the D-form [10]. However, when this chiral environment is lost by unfolding of αA-crystallin, protonation to the intermediate [I] could occur with an equal probability from both sides of the plane, resulting in an increase in L-Asp, and a D/L ratio of 1.0 [10]. In the present study, D-β-Asp residues were found at Asp-151 in LMW αA-crystallin and at Asp-58 in either LMW or HMW, while the Asp-151 of HMW αA-crystallin showed a low D/L ratio and an increase in β-Asp formation. The results are essentially the same as those found in cataractous and γ-ray irradiated αA-crystallin, suggesting that D-β-Asp formation may help stabilize the higher ordered structure surrounding the Asp residues. The function of the spontaneous stereoinversion may be to relieve the structural distortion within aged αA-crystallin.