![]() Figure 6 of
Reddy, Mol Vis 2002;
8:298-305.
Figure 6 of
Reddy, Mol Vis 2002;
8:298-305.
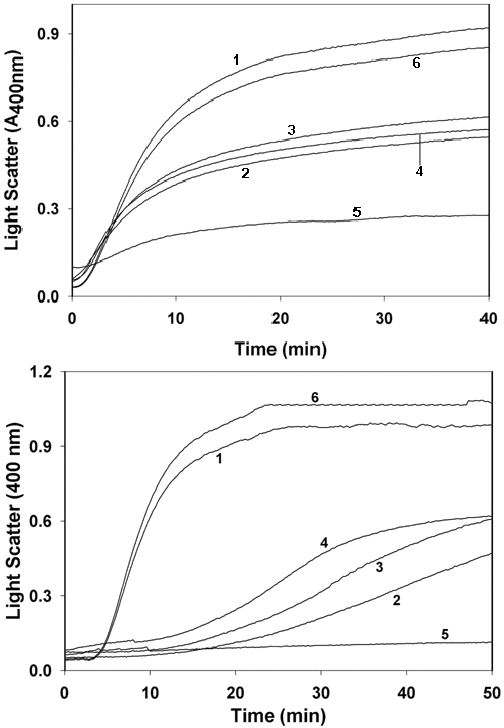
Figure 6. Chaperone activity of α-crystallin
A: Chaperone activity of α-crystallin as assessed by the suppression of DTT-induced aggregation of insulin B-chain. Insulin (0.4 mg/ml in 50 mM phosphate buffer, pH 7.2) was reduced with 20 mM DTT. Aggregation of insulin B-chain in the absence (trace 1) or in the presence of 0.5 mg/ml α-crystallin isolated from control (trace 2), food restriction (trace 3), protein restriction (trace 4), and vitamin restriction (trace 5) was monitored by measuring apparent absorption at 400 nm. Data are representative of three such independent assays for three separate lens extracts. Lysozyme (0.5 mg/ml, trace 6) did not prevent insulin aggregation. B: Chaperone activity of α-crystallin as assessed by the suppression of heat-induced aggregation of βL-crystallin. βL-Crystallin (0.3 mg/ml in 50 mM phosphate buffer, pH 7.2) was incubated at 65 °C in the absence (trace 1) or in the presence of 0.5 mg/ml α-crystallin isolated from control (trace 2), food restriction (trace 3), protein restriction (trace 4), and vitamin restriction (trace 5) group. Light scattering due to βL-crystallin aggregation was monitored by measuring apparent absorption at 400 nm. Data are representative of three such independent assays for three separate lens extracts. Lysozyme (0.5 mg/ml, trace 6) did not suppress βL-crystallin aggregation.