![]() Figure 3 of
Gross, Mol Vis 2000;
6:40-50.
Figure 3 of
Gross, Mol Vis 2000;
6:40-50.
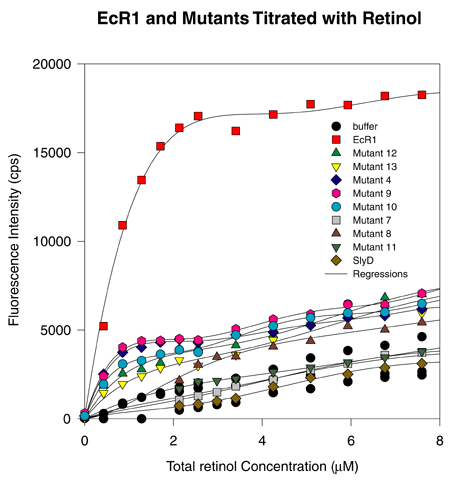
Figure 3. Retinol Fluorescence Enhancement of Mutants 4, 7, 8, 9, 10, 11, 12, 13, SlyD, and EcR1
Aliquots (1 mM) of EcR1, SlyD, or various mutants were titrated with retinol and the fluorescence enhancement of retinol measured. The abscissa represents total retinol concentration added to the cuvette. Fluorescence was recorded with a photon counting fluorometer and the units on the ordinate represent photons counted per second (cps). Each mutant protein has reduced retinol fluorescence enhancement activity compared to EcR1, suggesting that each mutant has an altered binding site for retinol. SlyD exhibited no fluorescence in excess of buffer alone, suggesting no saturable binding and little if any nonsaturable binding of retinal to SlyD.
There may be multiple classes of retinol binding mutants. For example, Mutants 4 and 9 exhibit roughly one-fifth the fluorescence enhancement of the wild type EcR1, while other mutants, for example Mutants 7 and 11, exhibit no detectable fluorescence enhancement. Other mutants seem to exhibit intermediate gradations of fluorescence enhancement. Thus, many different locations in the protein affect retinol binding, and the effects of a point mutation do not fall into a single class.