![]() Figure 3 of
El-Kabbani, Mol Vis 1999;
5:20.
Figure 3 of
El-Kabbani, Mol Vis 1999;
5:20.
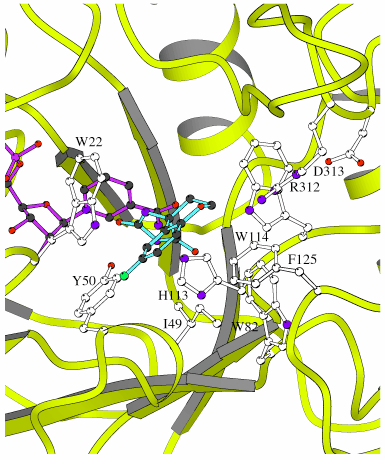
Figure 3. Inhibitor-binding site for porcine ALR1
Amino acid residues that interact with sorbinil are shown in white and labelled with residue type and number. Trp 22, Tyr 50, His 113, and Arg 312 form hydrogen bonds with the sorbinil molecule. The hydrophobic residues Ile 49, Trp 82, Trp 114 and Phe 125 are within van der Waals contacts with the inhibitor. A long hydrogen bond may exist between Trp 114 and sorbinil. The side chains of Arg 312 and Asp 313 engage in a salt link. Atoms of inhibitor (right) and coenzyme (left) are color-coded according to type (O is red, C is black, N is blue, P is pink, S is yellow and F is green). Ribbon drawings were prepared using MOLSCRIPT [25].