![]() Figure 3 of
Shih, Mol Vis 4:4, 1998.
Figure 3 of
Shih, Mol Vis 4:4, 1998.
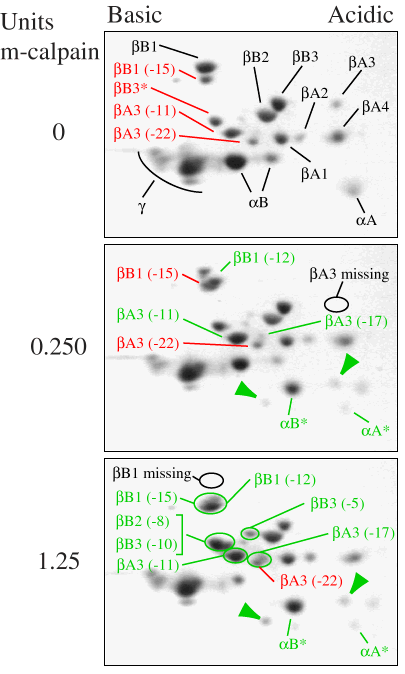
Figure 3. Digestion of water-soluble proteins from fetal bovine lenses with purified m-calpain
The digestion was performed for 10 min with either 0 units (top), 0.25 units (middle), or 1.25 units (bottom) of m-calpain. Following incubation, the appearance of truncated crystallins was observed by 2-DE. The identities of proteins in the sample incubated without m-calpain (top) were based on results of Edman sequencing as summarized in Table I. Partially truncated crystallins found in fetal lenses before m-calpain incubation are labeled in red, while intact crystallins are labeled in black. Partially degraded crystallins produced by m-calpain incubation (middle and bottom panels) are labeled in green. Regions where intact ßA3 and ßB1 were lost during incubation are indicated in the middle and bottom panels. The identities of truncated ß-crystallins produced by m-calpain digestion and the number of amino acids removed from their N-termini (given in parentheses) were determined by Edman sequencing summarized in Table III. The identification of C-terminally degraded [alpha]A- and [alpha]B-crystallins ([alpha]A* and [alpha]B*) was based on comparison to m-calpain digested rat [alpha]-crystallins [17]. The identity of the m-calpain degradation products marked with green arrows was not determined.