![]() Figure 2 of
Shih, Mol Vis 4:4, 1998.
Figure 2 of
Shih, Mol Vis 4:4, 1998.
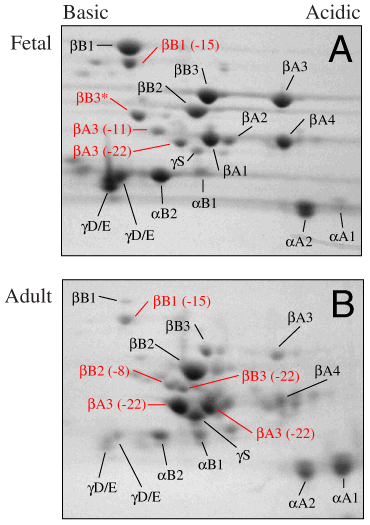
Figure 2. Two-dimensional electrophoresis of water-soluble proteins from fetal and adult bovine lenses
Identities of crystallins in fetal lens (A) were established by Edman sequencing shown in Table I. Undegraded crystallin subunits are labeled in black, and partially degraded crystallins are in red. The number of amino acids missing from the N-termini of the partially truncated ß-crystallins are shown in parentheses. The major truncated ß-crystallins in fetal lenses were ßB1 (-15), ßA3 (-11), and ßA3 (-22). The identities of ßB1, ßB1 (-15), ßB2, ßB3, ßA3, ßA4, [gamma]D/E, [alpha]B2, [alpha]B1, [alpha]A2, and [alpha]A1 in adult lenses (B) were assigned by comparison to the position of the corresponding proteins in fetal lenses. The identities of [gamma]S, ßB2 (-8), ßB3 (-22), and acidic and basic forms of ßA3 (-22) in adult lenses were determined by Edman sequence analysis as summarized in Table II. Intact ßB1, ßB3, ßA3, ßA4, [gamma]D/E, and [alpha]B decreased with age, while ßB2, and the two forms of ßA3 both lacking 22 amino acids from their N-terminus became major protein species. For reference, the calculated molecular weight and pI of acetylated bovine ßB1 are 28,054 and 7.13, respectively, and the calculated molecular weight and pI of acetylated bovine [alpha]A are 19,832 and 5.52, respectively. These images only show the region of the gels containing crystallins; the entire gel for Figure 2a is shown below.
For orientation, the uncropped two-dimensional gel of water-soluble protein from fetal bovine lens (Figure 2a) is also shown.