![]() Figure 4 of
Baer, Mol Vis 4:30, 1998.
Figure 4 of
Baer, Mol Vis 4:30, 1998.
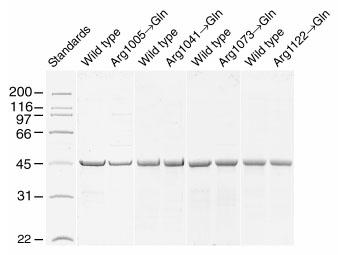
Figure 4. Coomassie blue stained SDS-10% polyacrylamide gels of purified recombinant X4IRBP and the four Arg->Gln mutants
In each panel X4IRBP was run along side one of the 4 Arg->Gln mutants (loading level = 5 µg). All of the mutants were expressed in E. coli as soluble thioredoxin fusion proteins with similar yields as X4IRBP. The proteins shown here were purified by ammonium sulfate precipitation followed by ion exchange chromatography. The purity ranged from 85 to 96% based on densitometric scans of the Coomassie blue stained gels. The concentration of the protein was determined by amino acid analysis.