![]() Figure 2 of
Ostrer, Mol Vis 4:28, 1998.
Figure 2 of
Ostrer, Mol Vis 4:28, 1998.
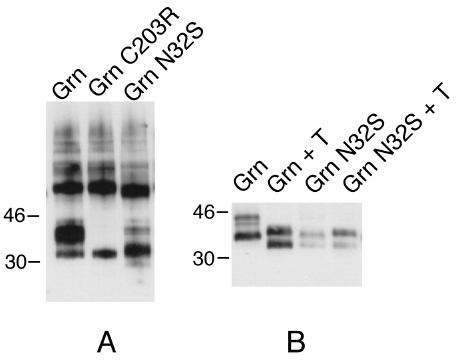
Figure 2. A western blot analysis of wild type and mutant green opsins
Polypeptides from lysates of equal numbers of cells were separated by 12% SDS-PAGE, transferred to nitrocellulose, and probed with 1D4 mAb. Immunoreactive bands were visualized using chemiluminescence (ECL) [12]. (A) The band that migrated just above the 30 kD molecular weight marker in the Grn-N32S lane represents the unglycosylated form. The band just below the 46 kd molecular weight maker in the Grn lane represents the fully glycosylated form. All bands above 46 kd represent multimeric forms of the protein. (B) Treatment with tunicamycin, Cells treated ± tunicamycin during the 16 hour induction with CdCl2. Although a minor band in the Grn-N32S lane was observed to co-migrate with the glycosylated form of green opsin, digestion of the opsin with endoglycosidases N and F did not eliminate this band, suggesting that it represented another form of post-translational processing.