![]() Figure 2 of
Howell, Mol Vis 4:15, 1998.
Figure 2 of
Howell, Mol Vis 4:15, 1998.
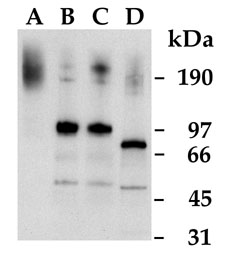
Figure 2. Western blot of human ciliary body proteins with rabbit antiserum against carboxy-terminal peptide of human placenta amine oxidase
Whole human ciliary body tissue was detergent-solubilized, subjected to SDS-PAGE, and analyzed by Western blotting with rabbit antiserum against the carboxy-terminal peptide of human placenta amine oxidase. Lane A, non-reduced sample. Lane B, reduced sample. Lane C, reduced sample mock-digested without glycosidase. Lane D, reduced sample digested with mixture of endoglycosidase F/N-glycosidase F. The faint bands at approximately 200 kDa in lanes B-D most likely represent a small amount of residual unreduced dimer (or dimers joined by linkages other than disulfide bonds). The lower molecular weight bands in lanes B-D may represent proteolytic degradation products; partial proteolytic degradation may also explain the molecular weight heterogeneity of the band in lane A. The antiserum does not crossreact with the flavoprotein amine oxidases MAO A and B; no bands of appropriate molecular weight for these enzymes (approximately 60 kDa for monomers, 120 kDa for dimers) are present in the blot.