![]() Figure 5 of
Lin, Mol Vis 3:17, 1997.
Figure 5 of
Lin, Mol Vis 3:17, 1997.
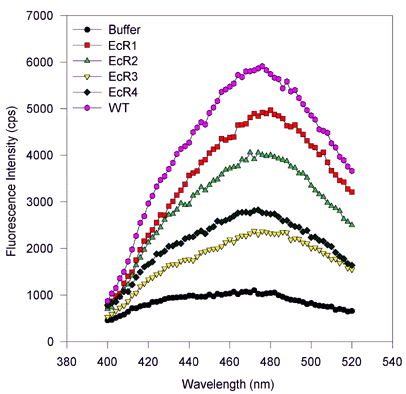
Figure 5. Fluorescence wavelength scans for retinol binding to variant and wild type IRBP.
(Excitation and emission scans, proteins expressed in baculovirus and E. coli.)
There are no substantial spectral shifts, and only the amplitude of the fluorescence varies from sample to sample. This suggests that only the number of binding sites varies from one mutant to the next, despite equimolar amounts of protein being scanned. In these scans, roughly 1 µM IRBP protein was mixed with 6 µM all-trans-retinol, after 100 s equilibration the wavelength scans were performed first holding the emission constant at 479 nm and varying the excitation wavelength from 250 to 400 nm. For emission scans the excitation was held at 339 nm, while the emission monochromator was varied from 400 to 550 nm. Measurements were made integrating over time for 2 s and measuring values every 2 nm. Slits were adjusted so that the bandpass for the emission and excitation monochromators was about 2 nm.
Figure 5a. Excitation scans, baculovirus-expressed proteins.
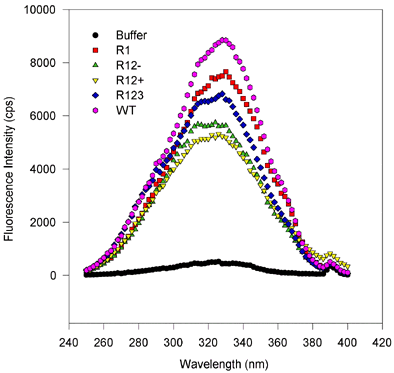
Figure 5b. Emission scans, baculovirus-expressed proteins
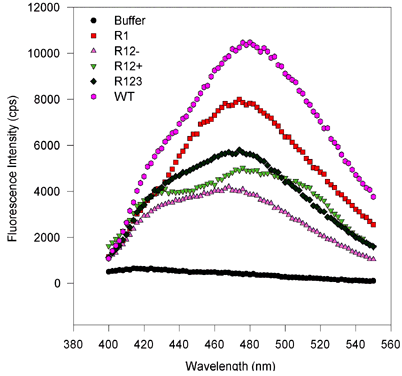
Figure 5c. Excitation scans, E. coli-expressed proteins
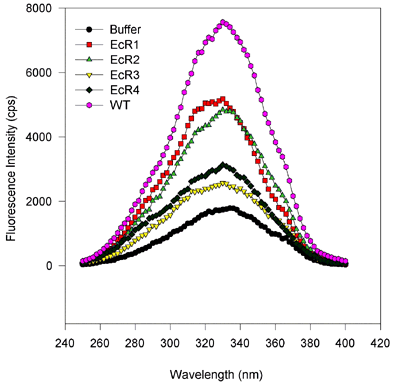
Figure 5d. Emission scans, E. coli-expressed proteins