![]() Figure 3 of
Lin, Mol Vis 3:17, 1997.
Figure 3 of
Lin, Mol Vis 3:17, 1997.
Figure 3a. Purity and Immunoreactivity of Wild Type IRBP and Variants.
SDS PAGE Analysis of protein content. The proteins were prepared as described in the text and analyzed on an 8% polyacrylamide gel. The gel was stained for protein with Coomassie R-250 and proteins from a duplicate gel were transferred to a membrane, which was immunostained with a monoclonal antibody against IRBP. Lane 1 shows molecular weight standards with myosin at 200 Kda, phosphorylase b at 97.4 Kda, bovine serum albumin, at 68 Kda, and ovalbumin at 43 Kda. Lane 2, wild type IRBP, 5 µg; Lane 3, G719S, 10 µg; Lane 4, R725C, 10 µg; Lane 5, R123, 10 µg; Lane 6, R12+, 20 µg; Lane 7, R12-, 10 µg; Lane 8, R1, 10 µg.
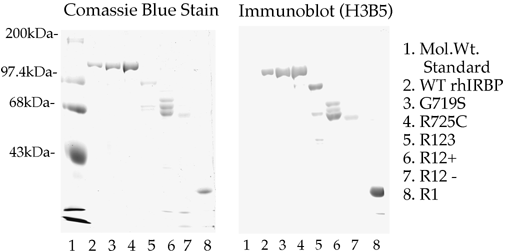
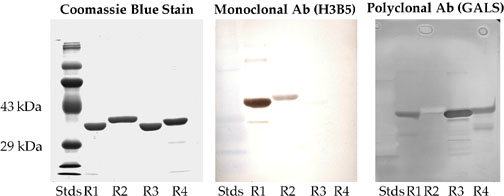
Figure 3b. Purity and Immunoreactivity of E. coli produced proteins.
Western blotting of E. coli expressed individual human IRBP repeats. Lane R1, EcR1, 3 µg; Lane R2, EcR2, 3 µg; Lane R3, EcR3, 3 µg; Lane R4, EcR4, 3 µg. The left panel represents the Coomassie R-250 stained gel. The central panel shows the corresponding western blot with the monoclonal antibody H3B5. The apparent staining of the molecular weight markers is an artifact. These markers were prestained blue in appearance. They could be seen as blue on the gel and could clearly be seen as blue on the blot after transfer, but before immunostaining. The immunoblots were developed with DAB and positive bands possess a characteristic brown reaction product that was clearly distinguishable from the blue-stained markers. The right panel represents an immunoblot with polyclonal antibodies (called GALS) against an oligopeptide (837-861) from Repeat 3. This panel did not employ prestained molecular weight markers. Pre-immune and nonimmune sera showed no DAB positive reactions. The immunoreactivity of the proteins shows that they are authentic and the correct size.