Figure 2. Schematic workflow for preparing 3D corneal organotypic cultures. Rabbit corneas were procured and processed on the day of
arrival approximately 24 h after enucleation. The corneas were punched centrally using a 9-mm trephine in a sterile Petri
dish containing phosphate buffered saline (PBS) with 1× penicillin-streptomycin antibiotic solution. The epithelial and endothelial
layers, along with the basement membranes (BMs), were removed under a dissection microscope using a scalpel blade. The corneal
stroma was cut into ~2-mm2 pieces and incubated overnight in an enzyme digestion medium containing collagenase and hyaluronidase. The stromal cell pellet
collected after a brief centrifugation contained the corneal keratocytes, which were incubated in a fibroblast medium containing
40 ng/ml of fibroblast growth factor (FGF-2). Once the desired confluency was reached, 50% of the cells were treated with
a myofibroblast medium containing 20 ng/ml of TGF beta-1, and the remaining 50% were maintained in the fibroblast medium.
The cells were mixed with collagen type-1 to prepare the cellular layer, which was loaded above the acellular layer and coated
onto the insert of six-well Transwell plates (Corning, NY). The cellular layer was incubated undisturbed for 4 days at 37°C
in 5% carbon dioxide (CO2). On Day 5, epithelial cells procured from commercial sources were prepared as a cell suspension and added to each insert,
which was then incubated for 2 days in epithelial medium I at 37°C. Subsequently, the media was removed from both the inside
and outside of the insert and replaced with fresh epithelial growth medium II only to the outside of the insert. The epithelial
cells were then exposed to the air–liquid interface for 18 days, and the medium was replaced every 2 days. At the end of 18
days of incubation, a 3D corneal construct with different combinations of stromal cells along with multi-layered epithelial
cells was generated.
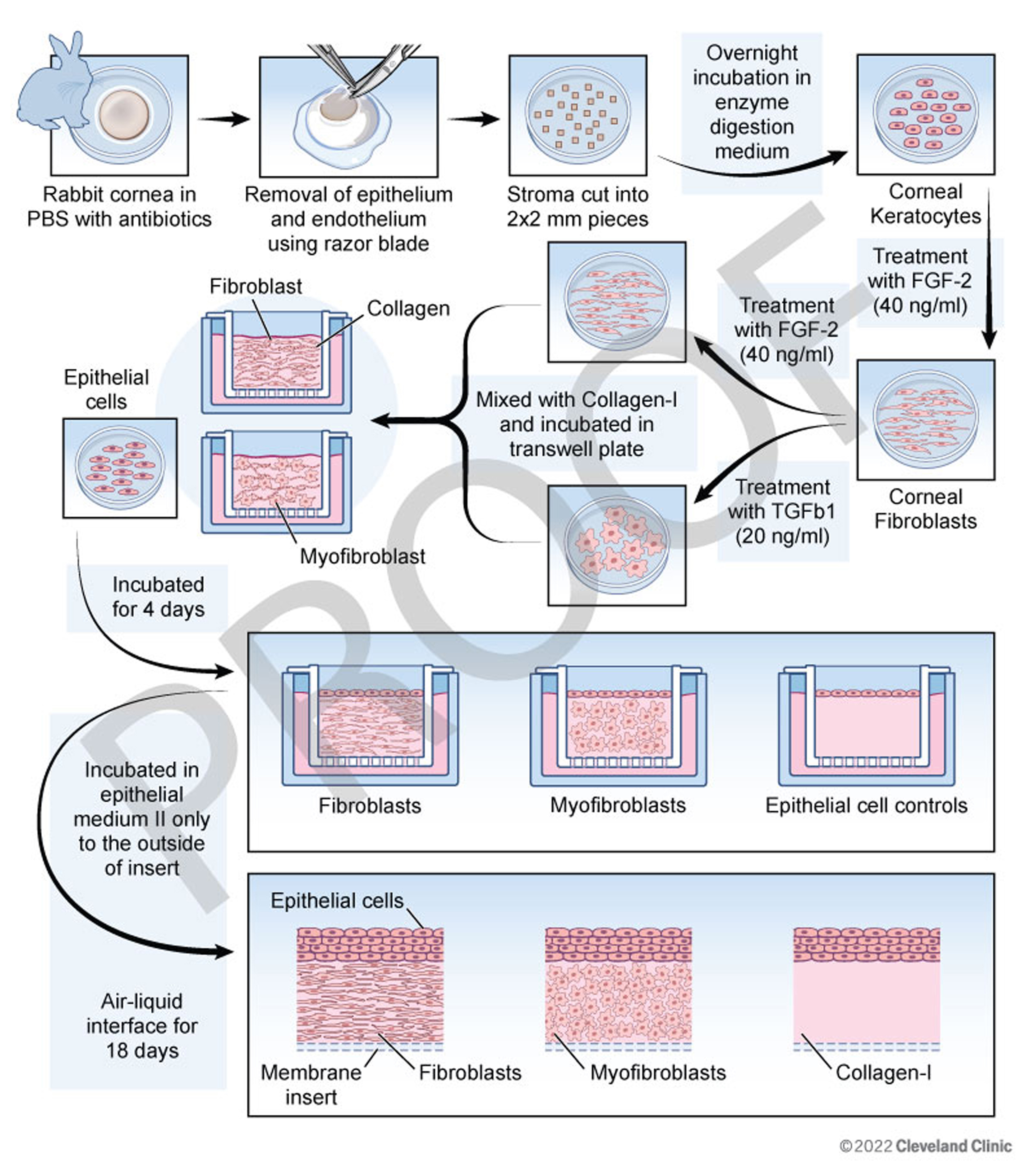
 Figure 2 of
Shiju, Mol Vis 2023; 29:68-86.
Figure 2 of
Shiju, Mol Vis 2023; 29:68-86.  Figure 2 of
Shiju, Mol Vis 2023; 29:68-86.
Figure 2 of
Shiju, Mol Vis 2023; 29:68-86. 