Figure 2. Encapsulated cell technology to deliver CR2-fH: Identification of a therapeutic dose. A: After systemic capsule delivery, CR2-fH was detectable in the RPE/choroid fraction of eyes with choroidal neovascularization
(CNV) lesions. A dot blot of RPE/choroid samples with twofold dilution steps is presented documenting a dose-dependent increase
in CR2 and CR2-fH delivery and binding to the tissues. B: CNV-induced complement activation in untreated animals was demonstrated by elevated levels of C3a when compared to animals
with no lesions (control). C3a levels remained elevated in animals exposed to CR2, and statistically significantly reduced
by CR2-fH. C: CNV sizes were reduced in animals injected systemically with ARPE-19 cells expressing CR2-fH as opposed to those expressing
CR2, with an apparent efficacious dose of 1,000 capsules. Data shown are average values (± standard error of the mean [SEM];
n = 2–3 animals per condition).
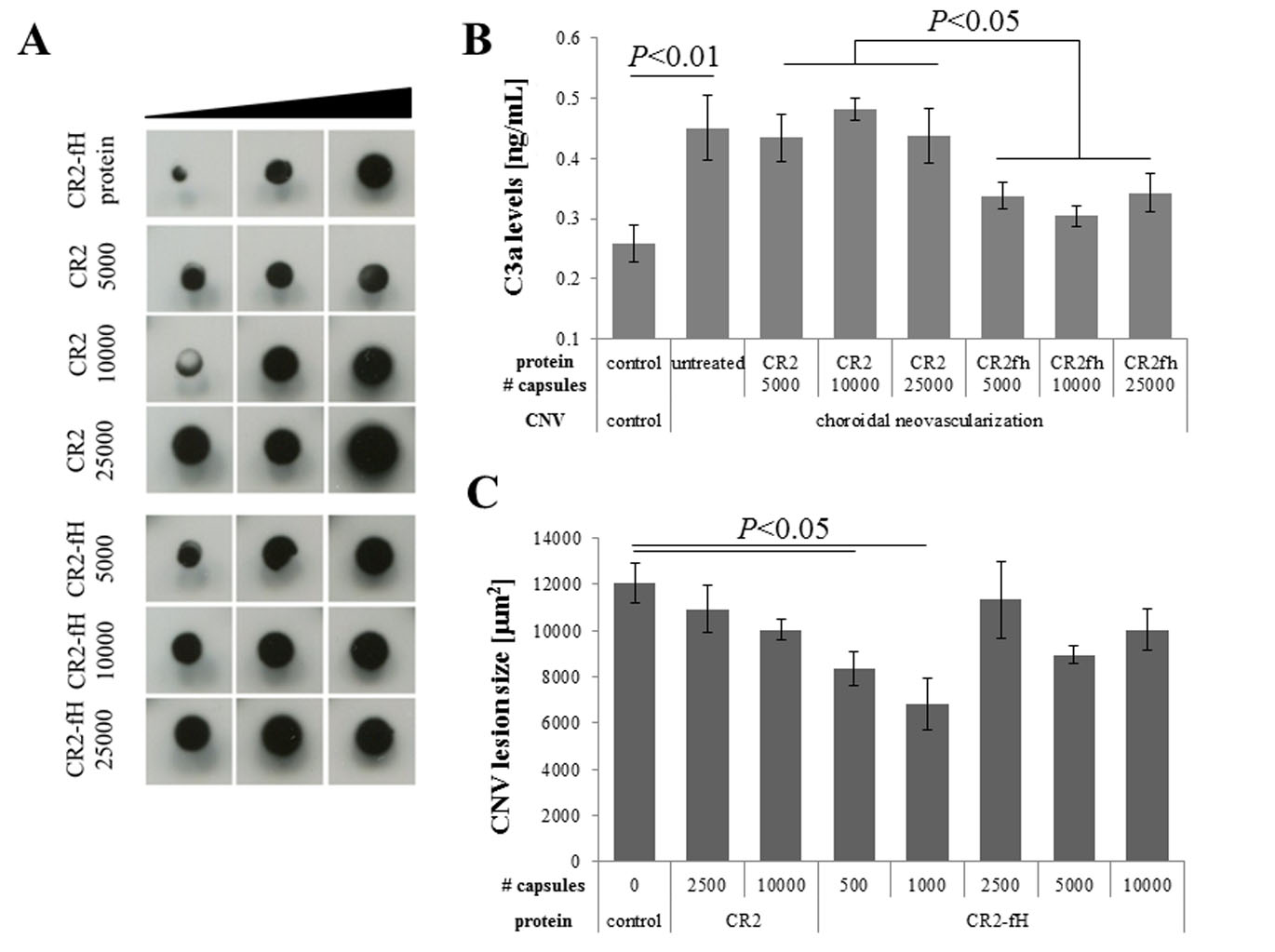
 Figure 2 of
Annamalai, Mol Vis 2020; 26:370-377.
Figure 2 of
Annamalai, Mol Vis 2020; 26:370-377.  Figure 2 of
Annamalai, Mol Vis 2020; 26:370-377.
Figure 2 of
Annamalai, Mol Vis 2020; 26:370-377. 