Figure 1. Encapsulated cell technology to deliver CR2-fH: Tool development. A: Detection of CR2-fH in the tissue surrounding the ARPE-19 cell-containing matrigel plug with immunohistochemistry using
an antibody against CR2. The corresponding differential interference contrast (DIC) and fluorescence image is presented. CR2
antibody staining was negative in control tissues containing empty alginate capsules. Scale bar: 100 μm. B: Systemically produced CR2-fH could trigger an immune response. Lack of immunoglobulin G (IgG) or IgM antibody production
was confirmed 1 month after capsule injection. Purified CR2-fH was run at two different concentrations and probed for the
presence of CR2-fH using the anti-CR2 antibody (positive control). Identical lanes were probed with serum from experimental
animals (S: CR2-fH) or control animals (S: control) at 1:50, followed by the appropriate secondary antibodies.
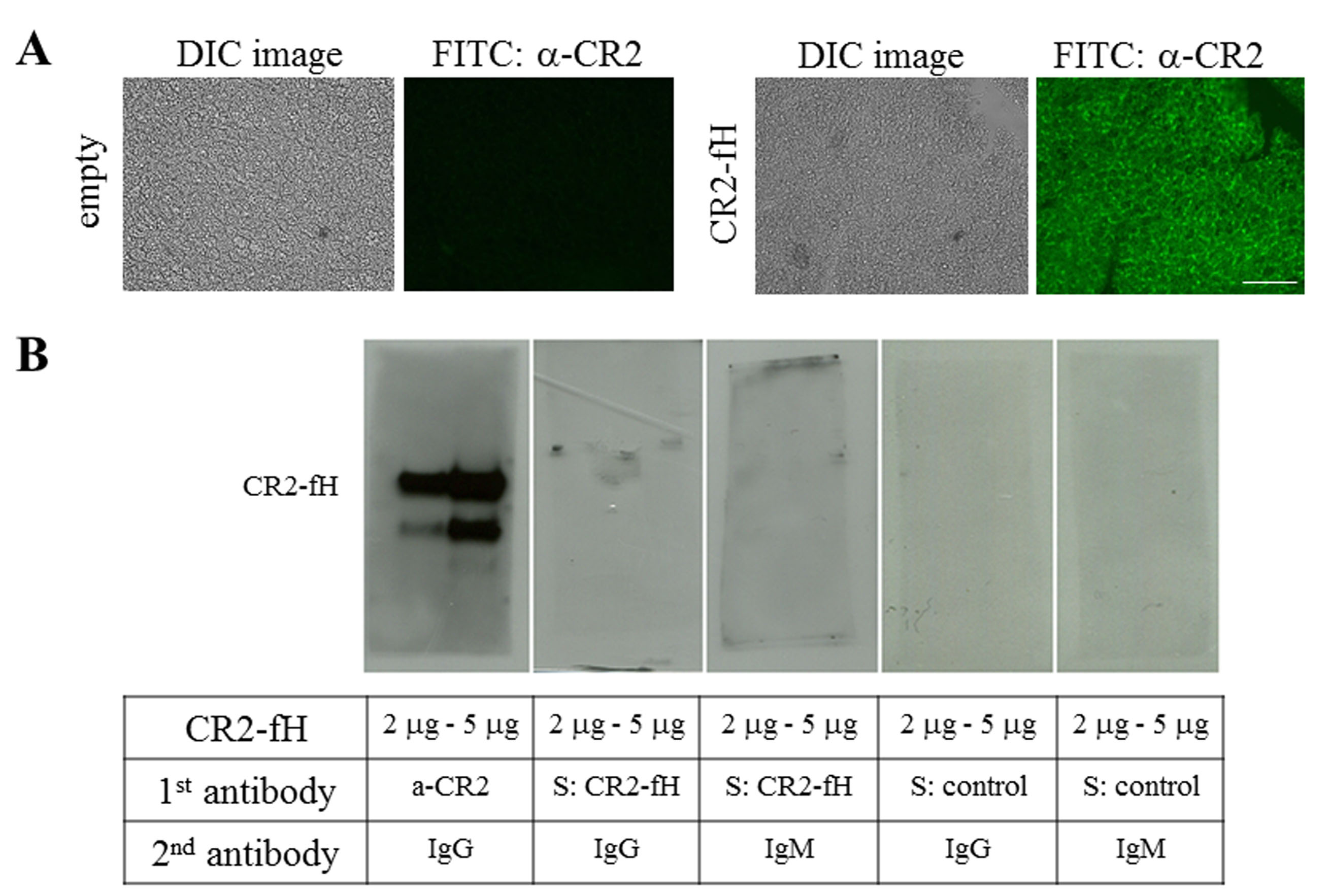
 Figure 1 of
Annamalai, Mol Vis 2020; 26:370-377.
Figure 1 of
Annamalai, Mol Vis 2020; 26:370-377.  Figure 1 of
Annamalai, Mol Vis 2020; 26:370-377.
Figure 1 of
Annamalai, Mol Vis 2020; 26:370-377. 