Figure 3. Biophysical characterization of the effect of the mutation in p.Q227X on the structures of βB1-crystallin. A: Far-ultraviolet (UV) circular dichroism (CD) spectra of the wild-type (WT) βB1 protein and its mutant protein. The spectra
were determined at room temperature using a JASCO spectropolarimeter, model J-810. The βB1-crystallin preparations at 0.5
mg/ml (50 mM sodium phosphate buffer, pH 7.4) were used to record the far-UV CD spectra. The path length was 0.1 cm during
the far-UV CD spectra determination. The spectra reported were the average of five scans, corrected for buffer blank, and
were smoothed. B: Intrinsic Trp fluorescence intensities of the WT-βB1 protein and its mutants. The proteins (0.2 mg/ml each) were dissolved
in 50 mM sodium phosphate buffer, pH 7.4, containing 100 mM NaCl, and were recorded with an excitation at 280 nm and emission
between 300 and 400 nm. C: Binding of 8-anilinonaphthalene-1-sulfonic acid (ANS) to the WT βB1 and mutant proteins. In these experiments, 15 μl of
0.8 mM ANS (dissolved in methanol) was added to a protein preparation (0.2 mg/ml, dissolved in 50 mM phosphate buffer, pH
7.4). The samples were incubated at 37 °C for 15 min before the fluorescence spectra were recorded after excitation at 380
nm and emission between 400 and 600 nm. D: Gel filtration chromatography of the WT βB1 and mutant (MU) βB1 proteins performed on an OHpak SB-804 HQ column.
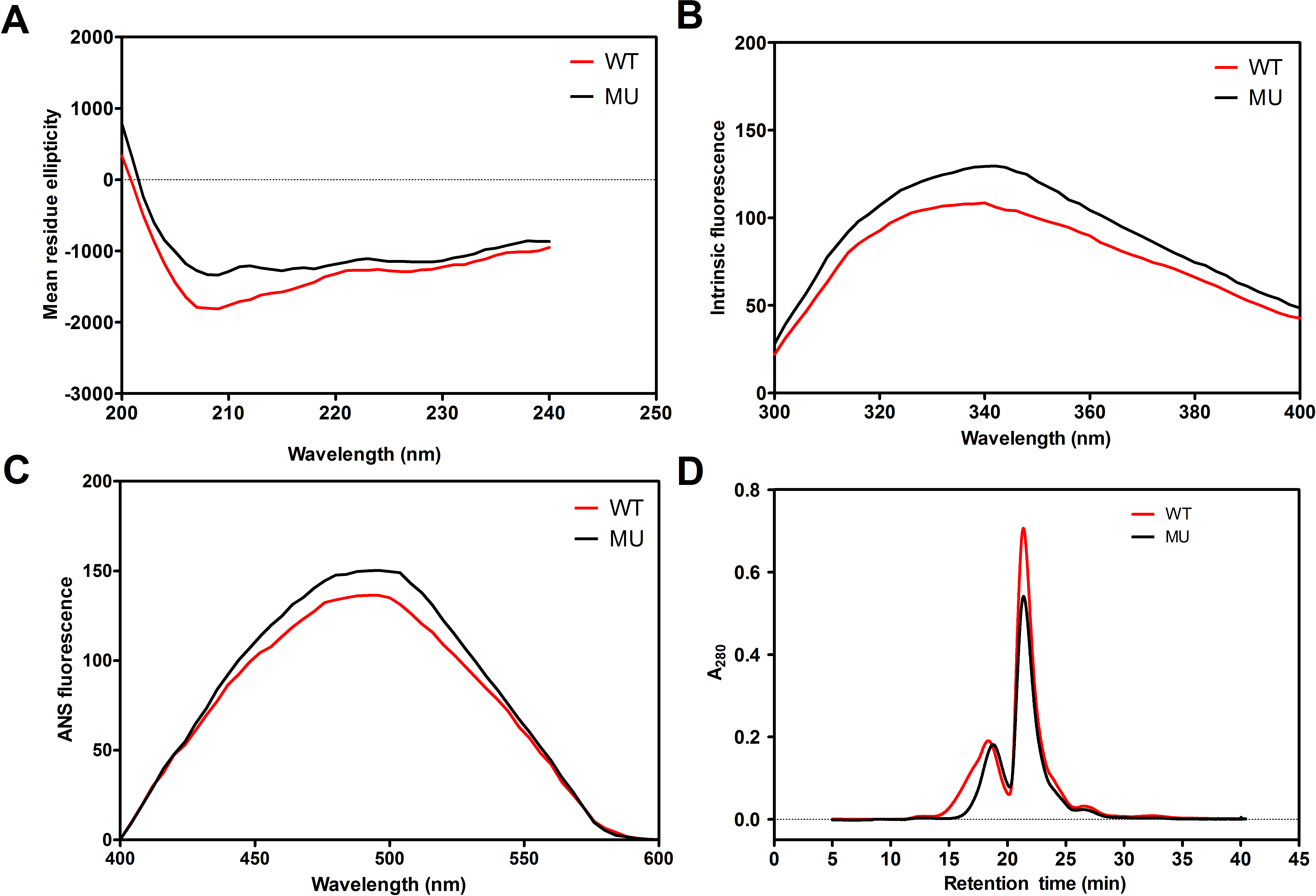
 Figure 3 of
Rao, Mol Vis 2017; 23:624-637.
Figure 3 of
Rao, Mol Vis 2017; 23:624-637.  Figure 3 of
Rao, Mol Vis 2017; 23:624-637.
Figure 3 of
Rao, Mol Vis 2017; 23:624-637. 