Figure 2. Modeling of the effects of the Arg343Trp mutation on TP63 structure. Beginning from a TP63 crystal structure, mutation of
residue 343 from Arg to Trp followed by side-chain repacking demonstrates a loss of favorable electrostatic contacts between
wild-type Arg and negatively charged phosphates of the accompanying double stranded DNA. This suggests that the functional
consequence of Trp is diminished binding affinity and thereby a change (i.e., a reduction) in transcriptional control. The
wild-type structure of TP63 (with an Arg residue at position 343) is shown in blue, the mutant structure with a Trp residue
at position 343 is shown in yellow, and a DNA helix is shown in brown and green.
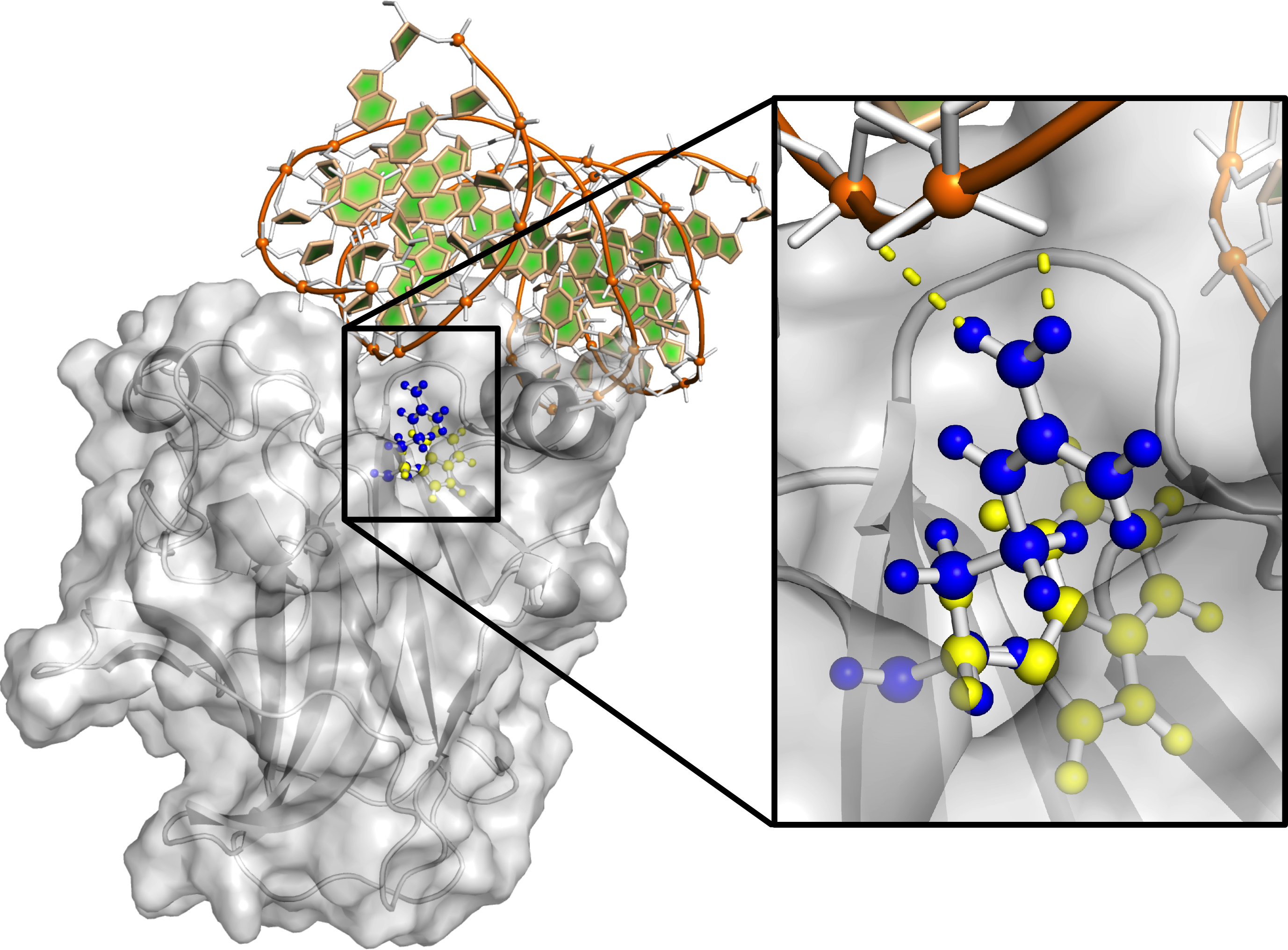
 Figure 2 of
Simpson, Mol Vis 2017; 23:179-184.
Figure 2 of
Simpson, Mol Vis 2017; 23:179-184.  Figure 2 of
Simpson, Mol Vis 2017; 23:179-184.
Figure 2 of
Simpson, Mol Vis 2017; 23:179-184. 