Figure 1. The G to W change at position 57 mildly affects the conformation of the protein. A: Far-ultraviolet (UV) circular dichroism (CD) spectra of wild-type CrygS and its mutant G57W. θ = ellipticity in millidegrees.
The protein concentration in each case was 0.2 mg/ml in 10 mM sodium phosphate buffer (pH 7.3). The cell path length was 2
mm, and all spectra were recorded at 27 °C, corrected for the background buffer signal. Each spectrum is an average of three
independent runs. B: Intrinsic fluorescence of wild-type and G57W CrygS. If = fluorescence emission intensity in arbitrary units; λexc = 295 nm. The protein concentrations used were 5 μM in 50 mM Tris-Cl, (pH 7.3). The cell path length was 3 mm, the excitation
and emission slits were 5 nm, and the spectra were recorded at 27 °C.
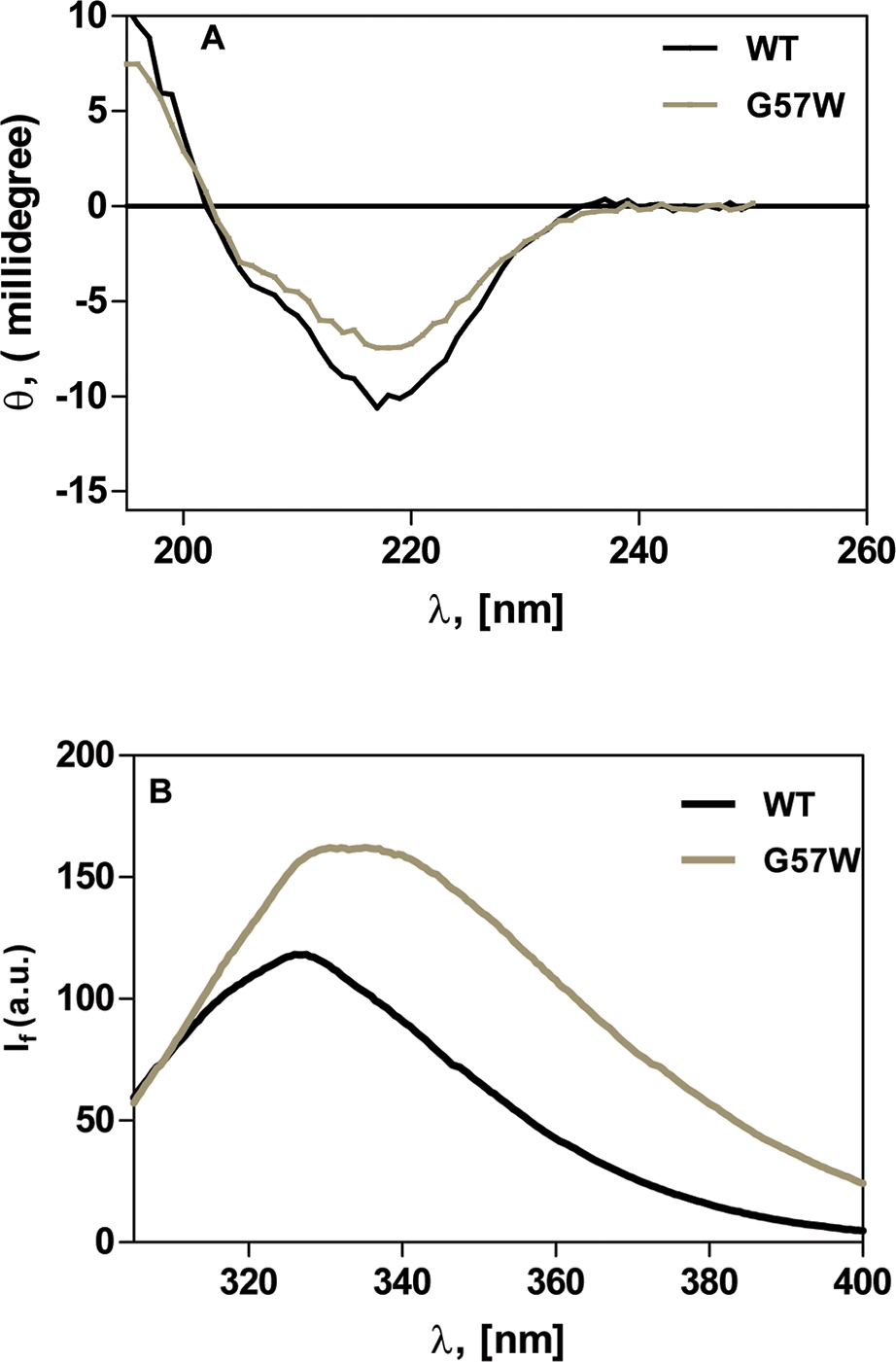
 Figure 1 of
Khan, Mol Vis 2016; 22:771-782.
Figure 1 of
Khan, Mol Vis 2016; 22:771-782.  Figure 1 of
Khan, Mol Vis 2016; 22:771-782.
Figure 1 of
Khan, Mol Vis 2016; 22:771-782. 