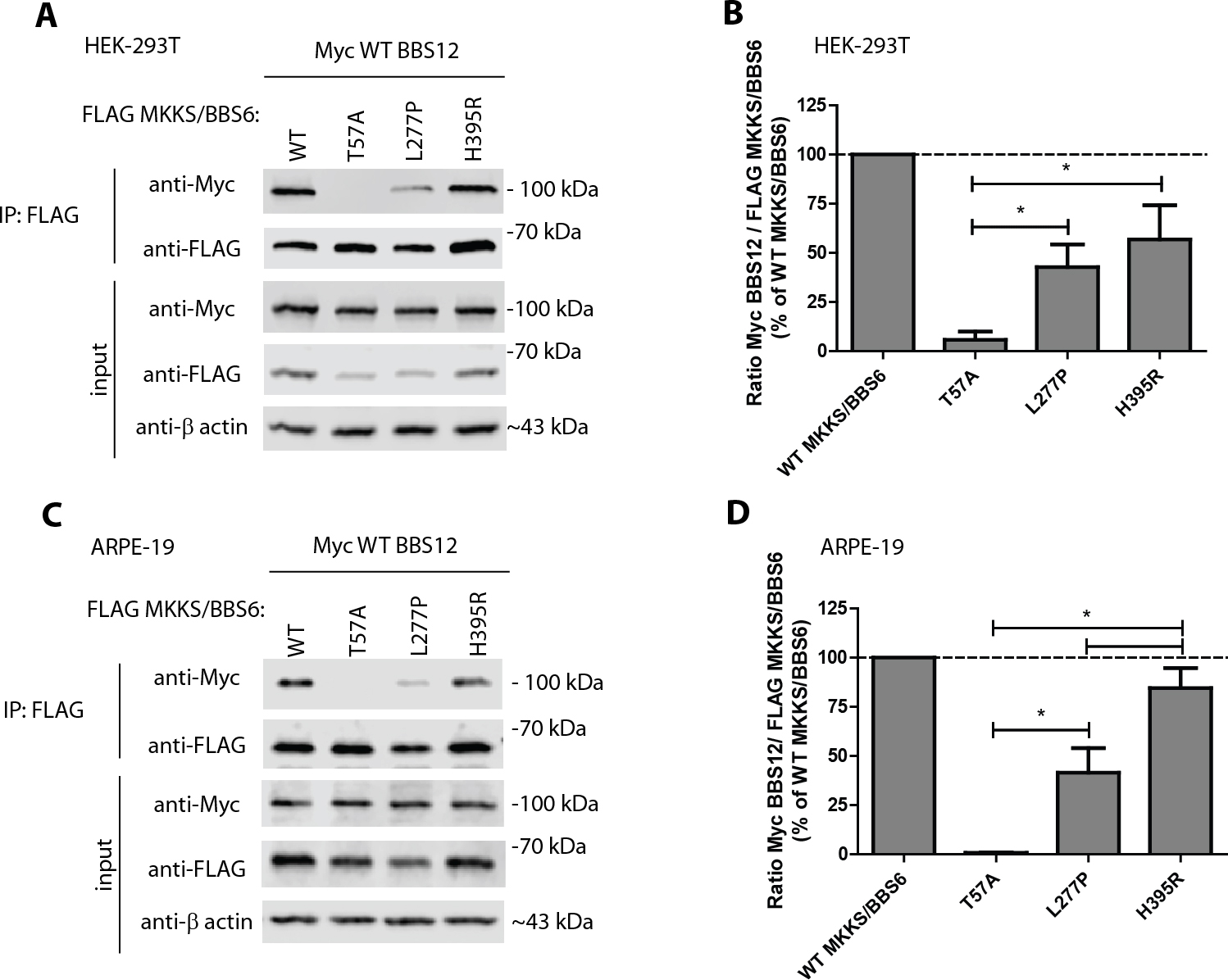
Figure 4. The H395R mutation differentially disrupts the MKKS/BBS6 interaction with BBS12 depending on the cell type used.
A: Evaluation of mutant MKKS/BBS6 interaction with BBS12. Human embryonic kidney (HEK-293T) cells were cotransfected with FLAG
MKKS/BBS6 variants along with Myc wild-type (WT) BBS12, and cell lysates were then subjected to immunoprecipitation (IP) using
anti-FLAG M2 beads as described previously [
7]. Representative data of more than three independent experiments.
B: Quantification of the MKKS/BBS6-BBS12 interaction described in (
A).
C: Mutant MKKS/BBS6 interaction with BBS12 in human adult retinal pigmented epithelium (ARPE-19) cells. ARPE-19 cells were
cotransfected with FLAG and Myc constructs as described above, and FLAG-tagged MKKS/BBS6 was immunoprecipitated. Representative
data of three independent experiments.
D: Quantification of the MKKS/BBS6-BBS12 interaction described in (
C). The band intensities of the IP’d Myc WT BBS12 and FLAG MKKS/BBS6 proteins were quantified using LI-COR software. The ratio
of Myc WT BBS12 to FLAG MKKS/BBS6 was calculated and displayed as a percentage of the Myc WT BBS12/FLAG WT MKKS/BBS6 ratio.
n≥3; average ±standard error of the mean (SEM); *=p<0.05,
t test. Note: The only variant that had a significantly different ability to bind to WT BBS12 in HEK-293T versus ARPE-19 cells
was H395R (p<0.05,
t test).
 Figure 4 of
Hulleman, Mol Vis 2016; 22:73-81.
Figure 4 of
Hulleman, Mol Vis 2016; 22:73-81.  Figure 4 of
Hulleman, Mol Vis 2016; 22:73-81.
Figure 4 of
Hulleman, Mol Vis 2016; 22:73-81.