Figure 1. Models for the progression of an R* molecule through successive states of phosphorylation and arrestin binding. Photoisomerization
generates the fully active state R*·P(0), representing non-phosphorylated metarhodopsin II. The C-terminus is successively
phosphorylated by rhodopsin kinase, and the number n of attached phosphate groups is indicated as R*·P(n). At each step, there is a possibility that arrestin can bind to create the form R*·P(n)·Arr, which is inactive. A: General case for phosphorylation and arrestin binding. The rate constants are unconstrained, and for state R*·P(n) the rate constants of phosphorylation and arrestin binding are denoted as ν(n) and μ(n), respectively; however, the single molecule can undergo only one of these two reactions. B: Arrestin binding is assumed not to occur until M = 3 phosphates have attached and then to occur with the same rate constant irrespective of how many phosphates have been
attached. For simplicity, the arrestin-bound states are not shown, as they are inactive. C: The truncated linear sequence to which the scheme in panel B is equivalent (see Theory). D: Alternative “three-state” scheme, in which the catalytic activity of phosphorylated R* drops to a lower level after M = 3 phosphates have attached and before arrestin binding. The rate constant of transition to this low-activity state is κ,
and the subsequent rate constant of arrestin binding is again μ. E: The truncated linear sequence to which the scheme in panel D is equivalent.
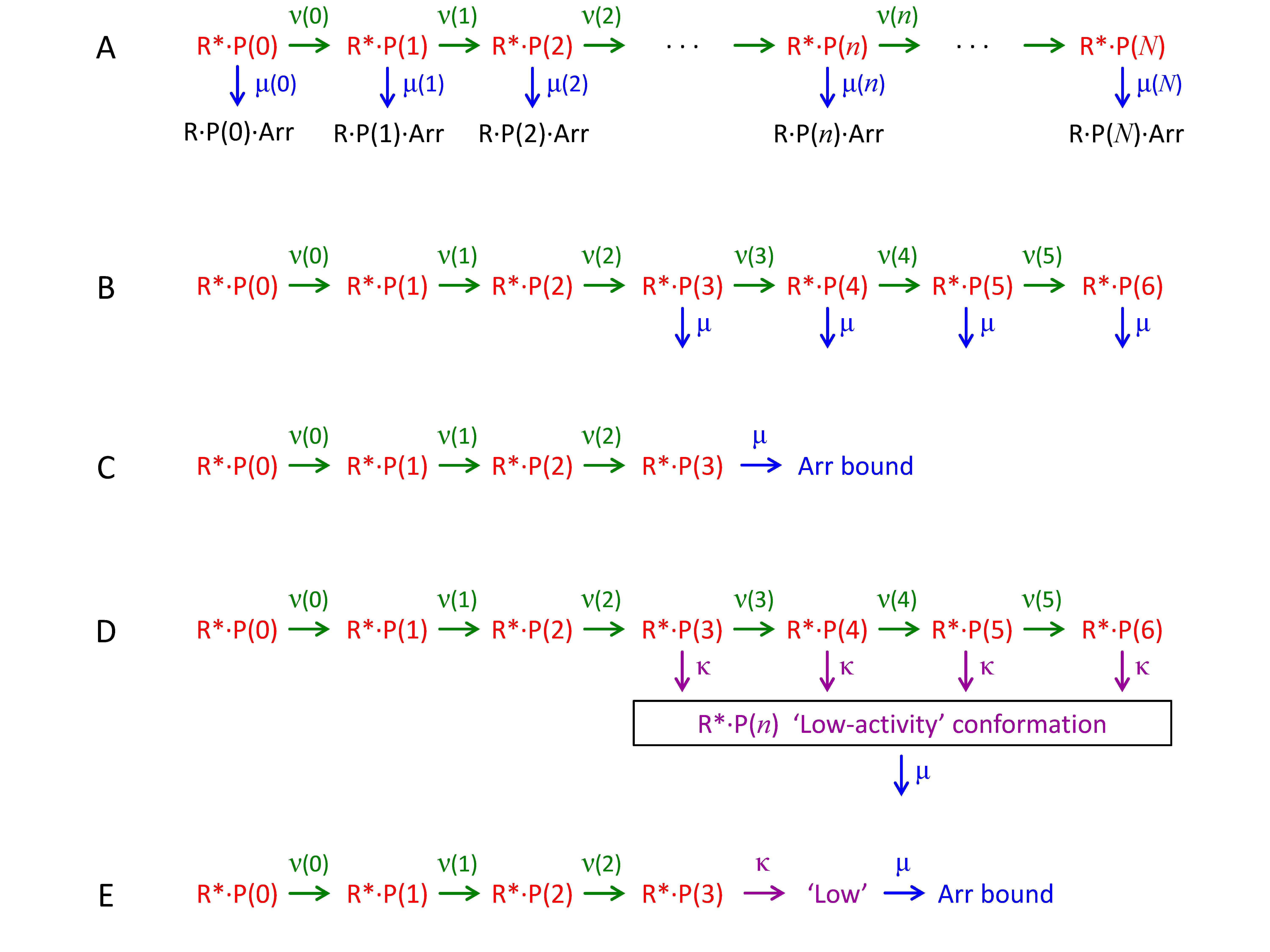
 Figure 1 of
Lamb, Mol Vis 2016; 22:674-696.
Figure 1 of
Lamb, Mol Vis 2016; 22:674-696.  Figure 1 of
Lamb, Mol Vis 2016; 22:674-696.
Figure 1 of
Lamb, Mol Vis 2016; 22:674-696. 