Figure 1. Pathologic events in CNV. A: In healthy cells, RPE cells have strong junctional complexes and form a monolayer on Bruch’s membrane separate from the
choroidal endothelial cells (CECs). B: Optical coherence tomography (OCT) of a human eye without age-related macular degeneration (AMD). Note the normal architecture
of retinal layers, lack of drusen, and good foveal contour. C: With increasing age and related to genetic predisposition, diet, smoking, oxidative stress, and inflammation, Bruch’s membrane
and the RPE extracellular matrix change in appearance with the formation of drusen and in composition, with deposition of
oxidized lipoprotein, debris, complement, and many other factors that incite inflammatory, oxidative, and angiogenic signaling.
Microglia are activated and release cytokines (e.g., tumor necrosis factor alpha, TNF-α) that stimulate RPE cells to express
vascular endothelial growth factor (VEGF). Concurrently, CECs activated to migrate toward the RPE monolayer and proliferate
into type 1 choroidal neovascularization (CNV). RPE cell–CEC contact initiates events that lead to RPE barrier compromise,
which permits cells and growth factors to move from basal to apical aspects. CECs are attracted to migrate across the RPE
monolayer and into the sensory retina toward a VEGF gradient to proliferate into type 2 CNV. D: OCT of a human eye with neovascular AMD, showing loss of the architecture of the retina and the RPE monolayer, loss of the
foveal depression, and cysts within the inner retinal layers.
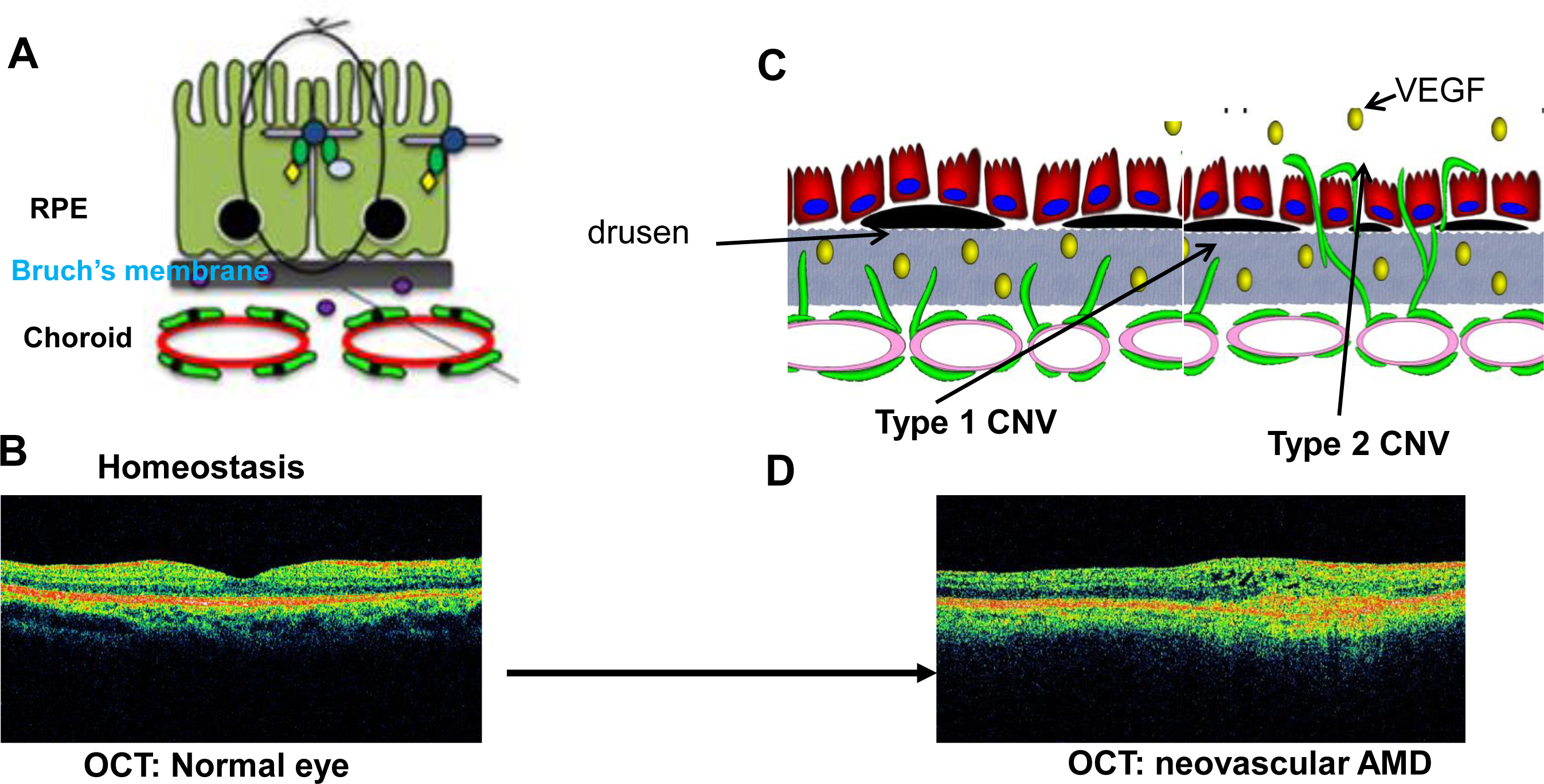
 Figure 1 of
Wang, Mol Vis 2016; 22:189-202.
Figure 1 of
Wang, Mol Vis 2016; 22:189-202.  Figure 1 of
Wang, Mol Vis 2016; 22:189-202.
Figure 1 of
Wang, Mol Vis 2016; 22:189-202. 