Figure 5. Molecular structure of Peptide 2. A: Representative conformers of Peptide 2 from the five highest-populated root-mean-square deviation (RMSD) clusters of the
molecular dynamics trajectory, with occupancies of 38%, 12%, 10%, 10%, and 9%. The peptide backbone is shown in a tube representation,
colored by the amino acid property type: gray for hydrophobic, green for polar, and brown for glycine. Cys2 and Cys12 are
marked as hydrophobic because they form a disulfide bridge. The PEG8 C-terminal extensions are shown in stick models of different colors for each conformer. The acetyl and amide terminal blocks
are shown in stick models, colored by atom type: gray for carbon, white for hydrogen, blue for nitrogen, and red for oxygen.
B: The major conformer of Peptide 2 (38% occupancy) in backbone tube representation, with key side chains in compstatin peptide
optimization in stick representation. The location of all amino acids is marked. The following color code is used: gray for
hydrophobic, green for polar neutral, blue for basic, red for acidic, yellow for cysteines and the disulfide bridge, and brown
for glycine. The PEG8-NH2 C-terminal extension is shown in stick representation without hydrogens, with carbons depicted in brown, oxygens in red,
and nitrogen in blue. C: Surface representation of Peptide 2, with the PEG8-NH2 extension shown in stick representation (including hydrogens). The color code is as in Panel B, with the added hydrogens of PEG8-NH2 shown in white. D: The chemical structure of PEG8-NH2.
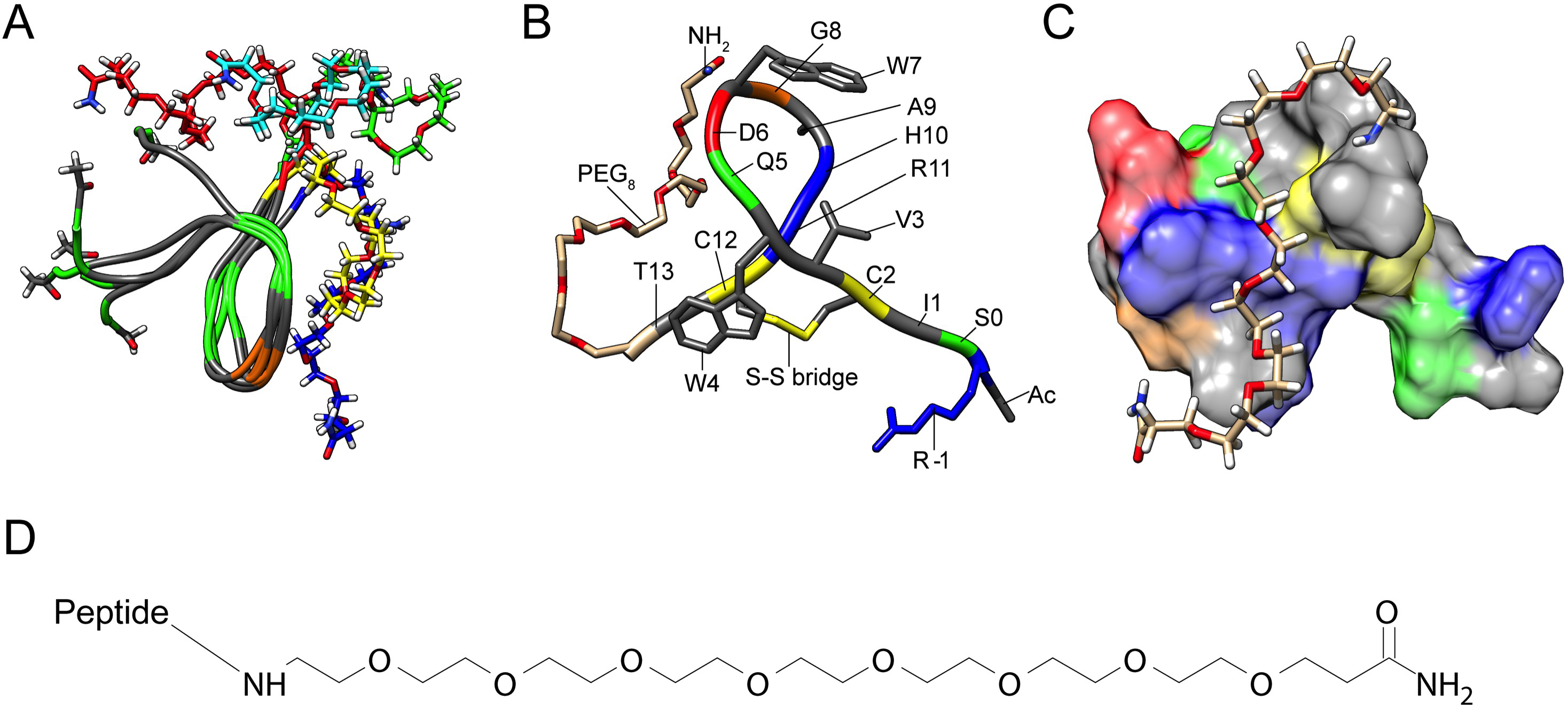
 Figure 5 of
Mohan, Mol Vis 2016; 22:1280-1290.
Figure 5 of
Mohan, Mol Vis 2016; 22:1280-1290.  Figure 5 of
Mohan, Mol Vis 2016; 22:1280-1290.
Figure 5 of
Mohan, Mol Vis 2016; 22:1280-1290. 