Figure 9. Hyperosmolarity induces NFAT5 gene and protein expression, and the DNA binding of NFAT5, in RPE cells. The mRNA level (A, B) was determined with real-time RT–PCR analysis in cells stimulated for 2, 6, and 24 h, and are expressed as folds of isoosmotic
unstimulated control. The protein levels (C-E) were determined by western blot analysis of cytosolic (C) and nuclear extracts (D, E) of cells stimulated for 6 and 24 h, respectively. A. Effects of osmolarity changes, CoCl2 (150 µM; n=5), and cell culture in 1% O2 (n=4) on the level of NFAT5 mRNA. The hyperosmotic media were made up by adding 100 mM NaCl (n=5) and 100 mM sucrose (n=3),
respectively. The hypoosmotic medium contained 60% of control osmolarity (n=5). Inset: Expression of β-actin and NFAT5 genes in RPE cells from different donors (1, 2) determined by RT–PCR. Negative controls (-)
were done by adding double-distilled water instead of cDNA as a template. B. Dose-dependent effect of high extracellular NaCl on the cellular level of AQP5 mRNA (n=4). The cells were cultured for 6
h in media that were made hyperosmotic by the addition of 10 to 100 mM NaCl. C. Effects of CoCl2 (150 µM), as well as of hyperosmotic (+ 100 mM NaCl) and hypoosmotic (Hypo) media, on the cellular level of the NFAT5 protein.
Similar results were obtained in three independent experiments using cells from different donors. D. Hyperosmolarity (+ 50 and + 100 mM NaCl, respectively) increased dose-dependently the nuclear level of the NFAT5 protein.
E. Hyperosmolarity (+ 100 mM NaCl) increased the nuclear levels of NFAT5 and p65/NF-κB proteins of RPE cells, while the nuclear
level of STAT3 protein remained unchanged. As a control, the nuclear level of the histone H3 (HisH3) protein was determined.
F. Hyperosmolarity (+ 100 mM NaCl) induces the DNA binding of NFAT5, as indicated by the appearance of complexes of NFAT5 protein
and labeled oligonucleotides in EMSA that were not observed under isoosmotic conditions. An excess of unlabeled oligonucleotides
(Competitor) abrogated the binding of NFAT5 protein to labeled oligonucleotides. Similar results were obtained in three independent
experiments using cells from different donors. Bars represent means ± SEM obtained in independent experiments performed in
triplicate. Significant difference versus isoosmotic unstimulated control: *p<0.05.
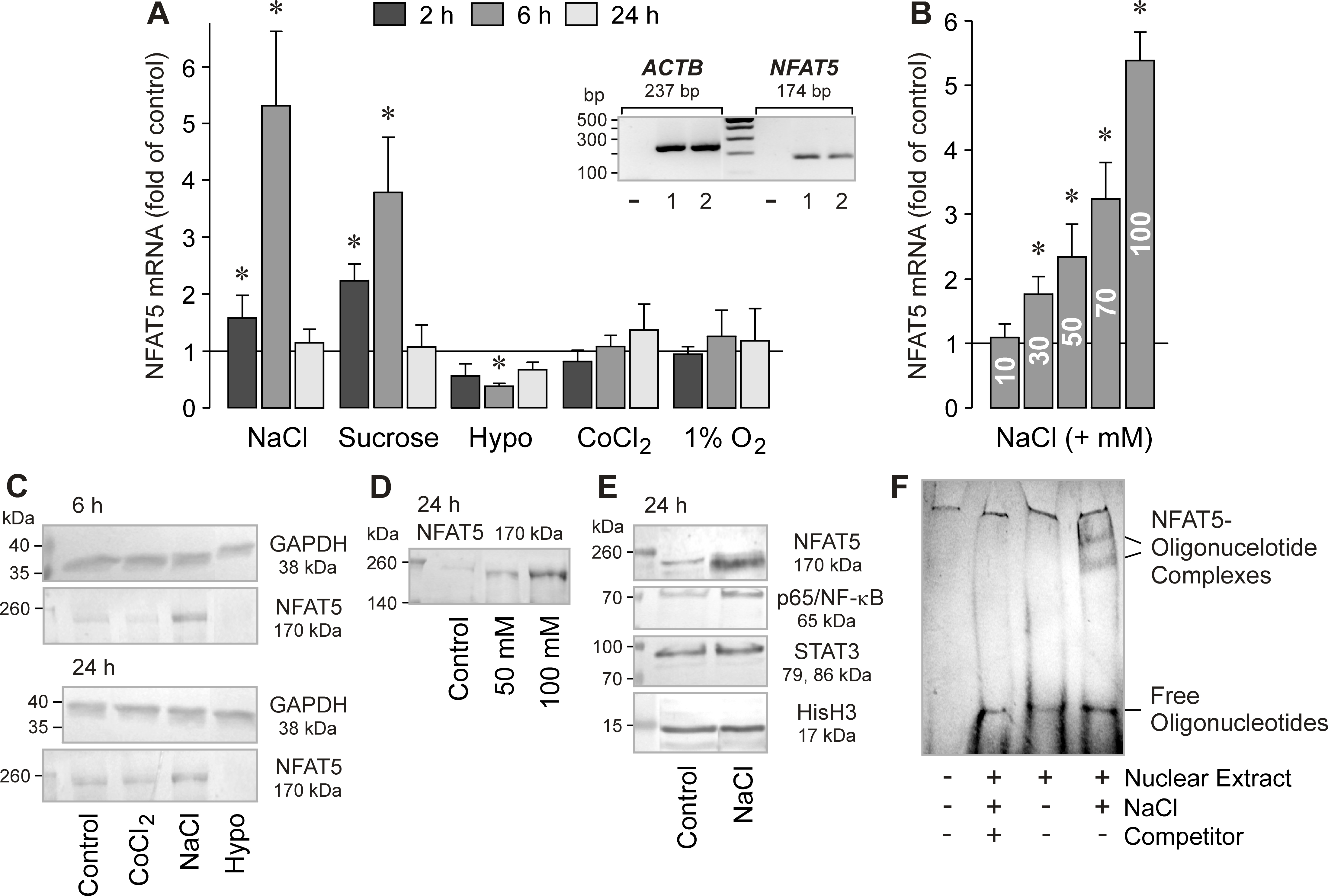
 Figure 9 of
Hollborn, Mol Vis 2015; 21:360-377.
Figure 9 of
Hollborn, Mol Vis 2015; 21:360-377.  Figure 9 of
Hollborn, Mol Vis 2015; 21:360-377.
Figure 9 of
Hollborn, Mol Vis 2015; 21:360-377. 