Figure 2. Ranibizumab effects on HTF’s viability in different concentrations and conditions (incubation time and culture media). A: Reduction in MTT absorbance in HTFs treated with ranibizumab at concentration 0.5 mg/ml in 5% FBS media conditions (continuous
24 h; p <0.05; n=3) *p <0.05 with respect to cells in media with 5% FBS with no ranibizumab treatment. B: Reduction in MTT absorbance in HTFs treated with ranibizumab at concentration 0.5 mg/ml in cells in serum-free media (continuous
24 h; p <0.05; n=3).*p<0.05 with respect to cells in serum-free media with no ranibizumab treatment. C: Reduction in MTT absorbance at 0.5 mg/ml ranibizumab in cells in media with 5% FBS. (continuous 48 h; p <0.05; n=3) *p<0.05
with respect to cells in media with 5% FBS with no ranibizumab treatment. D: Reduction in MTT absorbance at ranibizumab concentration of 0.05 mg/ml and 0.5 mg/ml in cells in serum-free media (continuous
48 h; p<0.05; n=3).*p<0.05 with respect to cells in serum-free media with no ranibizumab treatment.
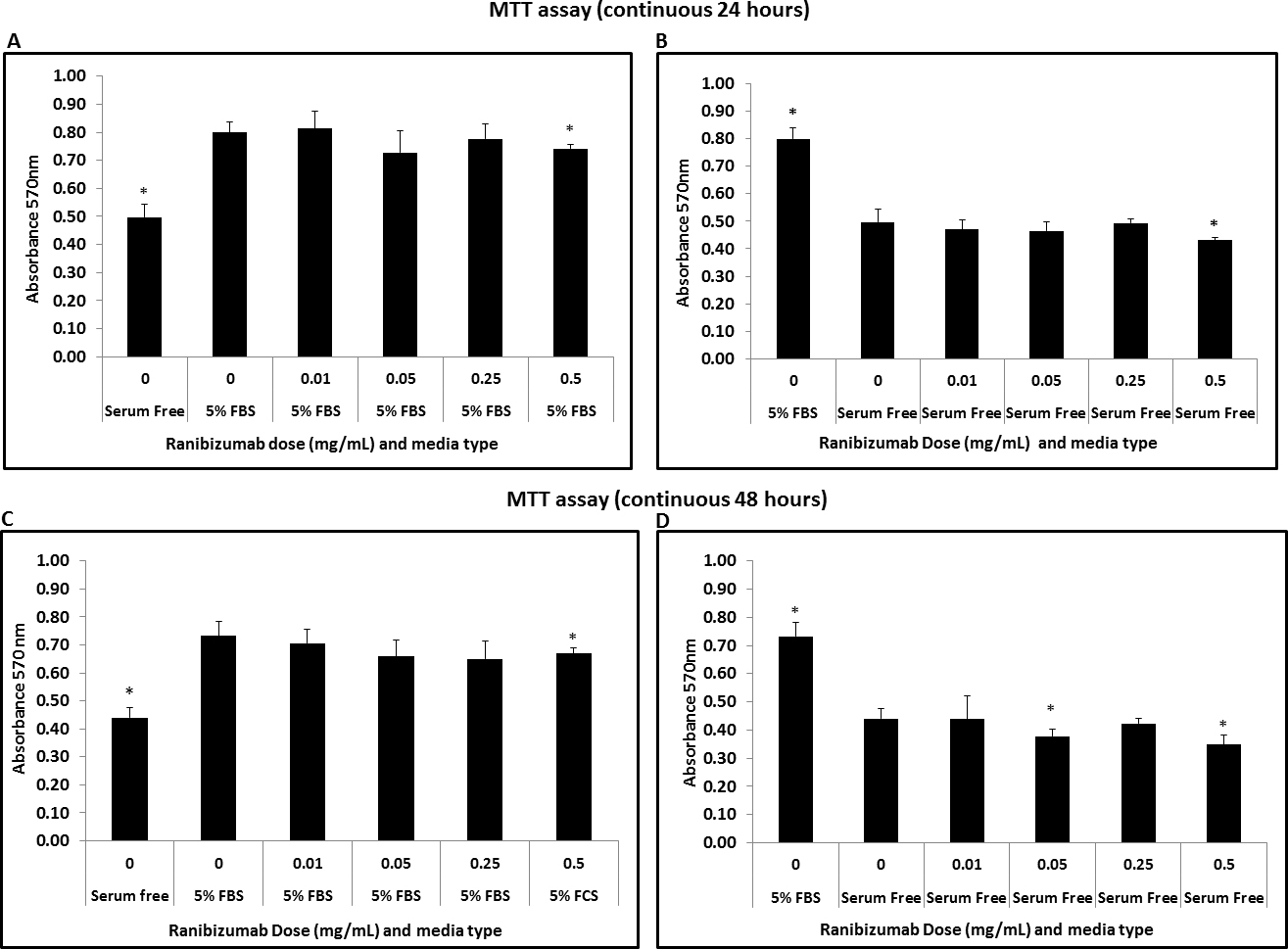
 Figure 2 of
Noh, Mol Vis 2015; 21:1191-1200.
Figure 2 of
Noh, Mol Vis 2015; 21:1191-1200.  Figure 2 of
Noh, Mol Vis 2015; 21:1191-1200.
Figure 2 of
Noh, Mol Vis 2015; 21:1191-1200. 