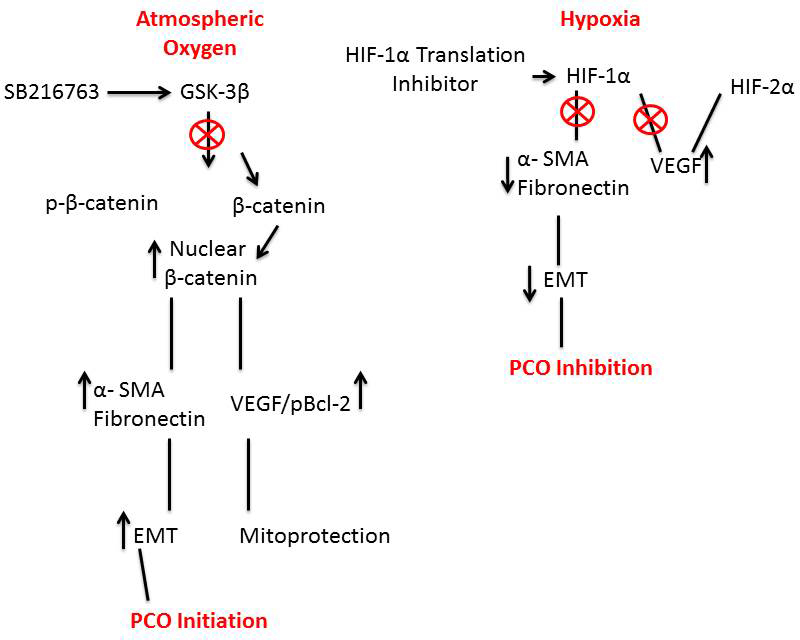
Figure 8. Schematic representation of the role of hypoxia inducible factor-1α or β-catenin on the regulation of expression of epithelial
to mesenchymal transition early marker proteins and VEGF synthesis. Atmospheric oxygen: Catalytic activity of glycogen synthase
kinase-3β (GSK-3β) is blocked using the specific GSK-3β inhibitor, SB216763. Inhibition of GSK-3β activity prevents the phosphorylation
of β-catenin. Non-phosphorylated β-catenin translocates to the nucleus. Nuclear β-catenin acts as transcription factor in
two independent pathways leading to [
1]; elevated synthesis and accumulation of fibronectin and alpha smooth muscle actin (α-SMA) and [
2] elevated synthesis of the prosurvival protein, vascular endothelial growth factor (VEGF). Increased accumulation of fibronectin
and α-SMA is an early marker indicator for epithelial to mesenchymal transition, which in the lens is a contributing factor
to posterior capsular opacification. At the same time, increased accumulation of VEGF promotes cell survival via the prevention
of mitochondrial depolarization and cell death. Although present in the cell, hypoxia-inducible factor-2α (HIF-2α) plays no
discernible role in these pathways under atmospheric oxygen conditions. Hypoxia: HIF-1α intracellular levels are decreased
using the specific HIF-1α translation inhibitor KC7F2. The resulting inhibition of the transcription factor HIF-1α suppresses
the synthesis of fibronectin and α-SMA, thus minimizing the trend toward epithelial to mesenchymal transition. Because of
the cooperation between HIF-1α and HIF-2α under hypoxic conditions, the suppression of HIF-1α is compensated for by HIF-2α
resulting in sustained VEGF synthesis and accumulation. Unlike the situation found with atmospheric oxygen, β-catenin appears
to play little to no role in these processes.
 Figure 8 of
Cammarata, Mol Vis 2015; 21:1024-1035.
Figure 8 of
Cammarata, Mol Vis 2015; 21:1024-1035.  Figure 8 of
Cammarata, Mol Vis 2015; 21:1024-1035.
Figure 8 of
Cammarata, Mol Vis 2015; 21:1024-1035.