In developing a model of slow light-induced retinal degeneration, ten miniature
pigs were submitted to constant lighting for a period ranging from one to three months.
Post-lighting survival time ranged from zero to two months. Control and illuminated animals were examined for pupillary reflex, underwent fundus examination and an electroretinogram. After euthanasia, retinas were processed
for histology with measure of outer nuclear layer thickness. All animals illuminated
one or more months had pupillar reflex alteration. Mean outer nuclear thickness was
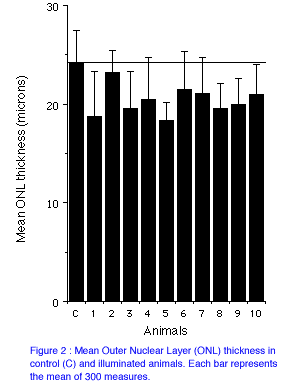
24.12 microns in the control and ranged from 18.36 to 21.45 microns in illuminated animals
(mean reduction 20%). Despite the pigmentation of miniature pigs, consistent results were obtained
in the absence of pharmacologic pupil dilation.
One of the main animal models of acquired retinal degeneration is the effect of constant light, described in the rat (1). Exposing rats to constant light (24 hours a day) leads to rapid photoreceptor degeneration with outer nuclear layer (ONL) thinning. This model has been shown to be especially effective in albino rats, whose photoreceptors are destroyed after some days at moderate intensity levels (2). The exact mechanism remains unknown, although the primary lesion probably lies in the photoreceptor outer segment, at the phototransduction level (as in retinitis pigmentosa), and may involve oxidative mechanisms with free radical liberation (3). Besides the rat, light-induced retinal degeneration has been described in monkeys (4, 5), rabbits (6), and pigeons (7).
The Yucatan miniature pig is used for experimental surgery protocols because
of its small size (adults weight about 30 kg) and easy manipulation (8, 9). The size,
anatomy and histology of its eye are much closer to the human eye than rodent eyes. The major features of the miniature pig's retina are: the large
size of photoreceptors and horizontal cells, the thickness of the nerve fiber layer
corresponding to numerous astrocytes, the presence of some rods in the foveola and
the absence of central avascular zone, with arterioles persisting in the foveola. Cones
are distributed throughout the retina with a proportion of approximatively 1 cone
to 7 rods (10, 11). The size of the globe, lens and cornea lends feasibility to
vitreoretinal surgery and experimental procedures such as retinal transplantation (retinal pigmented
epithelium and/or photoreceptors) and local gene therapy. However, no retinal degeneration model exists in the miniature pig. The aim of this study is the preliminary development of such a model by a constant light procedure.
Materials and Methods
Ten adult male Yucatan miniature pigs aged one year and two control animals were used (Charles River, Cleon, France). During lighting, animals were maintained in a 20 m2 room (white walls and ceiling) without movement restriction. Neon tubes situated 2.5 m above animals provided constant light (spectrum 400-700nm), with a 2500 lux intensity measured at the level of the animals' heads. Room temperature was 20oC, food and water were furnished ad libitum.
Before and after the constant light exposure experiments, animals were exposed to 12 hours of light per day, intensity 1000 lux, as were control animals. Lighting time and post-lighting survival time are presented in Table 1.
Animal Lighting Survival Control 0 0 1 1 12 2 2 10 3 4 0 4 4 4 5 4 6 6 8 0 7 8 4 8 8 8 9 12 0 10 12 4
At the beginning of light exposure and each week until sacrifice, each animal was examined for pupil size and reactivity and a fundus examination conducted. Animals n°8, 10, and one control animal had electroretinographic assessment under general ketamine anaesthesia (Pantops, Biophysic Medicals) just before sacrifice (white and blue light stimulation, left and right eye separately). At the end of survival time, animals were sacrificed by a pentobarbital overdose. Eyes were enucleated and immediately processed for histologic examination. Animals were treated in accordance with the ARVO statement for the use of animals in ophthalmic and vision research and protocol was approved by a committee on ethics.
Following experimental manipulation, the entire right eye was fixed in Bouin's fixative for three days, the anterior segment (cornea, iris, lens, ciliary body) was then removed and the posterior segment divided into five regions: Posterior pole (including optic nerve head and macula), nasal, temporal, superior and inferior regions. Specimens were washed for two days, embedded in parafin and microtomy was executed strictly perpendicularly to the retinal surface. Every 200 microns, a 10 microns thick section was selected and 20 sections were kept for each retinal region. Sections were stained with hematoxylin. Outer nuclear layer (ONL) thickness was measured by an image analyzing system (Biocom, Les Ulis, France). Three measures were performed for each section, regularly spaced, so that 60 measures were made for each retinal region and 300 measures per individual retina. The ONL thickness measurement appears to be the most convenient method to evaluate photoreceptor cell damage quantitatively (12). The left eye was embeded in OCT for further immunohistochemistry studies.
Results
For light exposure times greater than one month, animals weight was reduced by about 20%. Even in control animals, pupil size remains large and illumination did not lead to a narrow myosis. Fundus examination was always possible without dilation. After two months, the pupillary reflex appeared weak and slow when compared to control animals. Fundus examination showed no difference between control and illuminated animals and the post mortem macroscopic appearance of the retina (pigmentation, disc, vasculature) was the same in all animals.
Electoretinogram was, in all cases, normal in morphology and amplitude (a and b waves, white and blue light), without difference between control and illuminated animals (data not shown).
Figure 2 illustrates ONL thickness (mean of five retinal regions) for control and illuminated animals. Mean thickness was 24.12 microns in control animals and ranged >from 18.36 to 21.45 in the illuminated animals (except in animal n°2), suggesting a reduction of approximately 20%.

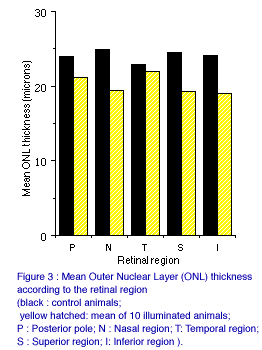
Considering the different retinal regions, Figure 3 shows ONL thickness for control and illuminated animals (mean of animals 1 to 10) in the posterior, nasal, temporal, superior and inferior regions. The effect of prolonged lighting appeared to be especially marked in the nasal, superior and inferior region, less in temporal region and intermediate in the posterior pole.
Figures 4 through 36 illustrate the microscopic aspect of the posterior, superior and inferior retina in control and illuminated animals. Generally, the number of cell layers was reduced from six or seven to three or four and the thickness of the layer often became irregular, so that ONL thinning sometimes appeared focal. The artifacts brought by the fixation technique did not allow a good assessment of the outer segments.
Appendix A contains a form that returns any combination of Figures 4 through 36 for comparison.
Appendix B contains a table of the ONL thickness for every section.
Discussion
The main point of this study is the use of a pigmented animal, whose eye is similar to the human eye. Rats used by researchers for retinal degeneration studies are generally albino animals, without pigmentation of the iris or the retinal pigment epithelium. The hypothesis has been advanced (1) that pigmentation protects from light degeneration. In fact, intrinsic retinal sensitivity to light exposure doesn't appear to be influenced by pigmentation, but the amount of light reaching the photoreceptors is diminished in pigmented animals because light is partially stopped by the iris (13, 14). Consequently, light-induced retinal degeneration has been obtained in pigmented rats only when pupils were dilated (13). The effect observed in undilated pigmented miniature pigs can be explained by the fact that, in this animal, pupil size remains relatively large even when illuminated (about 5 mm). In the rat, pupil reactivity is much stronger, leading to a very small pupil (less than 1mm), and the amount of light reaching the retina is greatly diminished. The effects observed after illumination times up to three months are much milder and slower in development than in albino rat retina. We think this is important since human disorders generally evolve over many months or years, with very slow photoreceptor degeneration.
The presence of a degenerative effect in animal n°1 (one week of lighting) and not in animal n°2 (two weeks of lighting) suggests individual variations for short illumination times. The effects were consistent for all animals illuminated for one month or more.
The normality of electroretinographic assessment in either control or illuminated animals could be related to the fact that only 20% of the ONL is damaged. Additionally, illuminated retinas showed normal macroscopic features. Electroretinograms are thought to reflect the functionality of total retinal tissue, and are only affected with severe and extended retinal injury.
Concerning the histological aspect of light damaged retinas, the lesions, as in the rat, principally involve the photoreceptor cells, manifested as reductions in the cell layers and disorganizations of outer segments. Inner retinal layers exhibited more discrete alterations. The regional distribution of lesions, classically predominant in the superior region (13), is not the same here. The major feature is the relative preservation of temporal photoreceptors. One hypothesis to explain this could be the anatomical disposition of pig's eye relative to the nose, with differential access of the light to the temporal retina.
Light-induced retinal degeneration, with primary lesion probably involving the
phototransduction molecules, can be compared to the worsening effect of light on
age-related macular degeneration and to the genetic defects of different phototransduction
proteins in retinitis pigmentosa. The present model of slow light-induced retinal
degeneration in the Yucatan miniature pig could be useful for evaluation of preventive
molecules, as previously described in the rat, such as ascorbate (15), dimethylthiourea
(16), NO synthase inhibitor (17), cytokines, and growth factors (18, 19). Retinal transplantation
and gene therapy could also be tested in this animal whose eye resembles the human
one, with realistic possibilities of experimental vitreoretinal surgery in eyes affected by a chronic retinal degeneration.
Acknowledgements
This work was supported in part by Fondation pour la Recherche Medicale.
References
1. Noell WK, Walker VS, Kang BS, Berman S. Retinal damage by light in rats. Invest
Ophthalmol 1966;5:450-473. ![]()
2. LaVail MM: Eye pigmentation and constant light damage in the rat retina; in: Williams TP, Baker B (eds): The Effects of Constant Light on Visual Processes. New York, Plenum Press, 1980, 357-387.
3. Organisciak DT, Winkler BS. Retinal light damage: Practical and theoretical considerations; in: Chader G, Osborne N (eds): Progress in Retinal Research Volume 13. New York, Pergammon Press, 1994, 1-29.
4. Tso MOM. Photic maculopathy in rhesus monkey : A light and electron microscopy
study. Invest Ophthalmol Vis Sci 1973;12:17-34. ![]()
5. Tso MOM, Woodford BJ. Effect of photic injury on the retinal tissues. Ophthalmology
1983;90:952-963. ![]()
6. Lawill T. Efects of prolonged exposure of rabbit retina to low-intensity light. Invest Ophthalmol Vis Sci 1973;12:45-51.
7. Marshall J, Mellerio J, Palmer DA. Damage to pigeon retinae by moderate illumination
from fluorescent lamps. Exp Eye Res 1972;14:164-169. ![]()
8. Lane C, Boulton M. Retinal pigment epithelial transplantation: technique and possible applications; in Brunsmann F, Von Gizycki R (eds): Retinitis Pigmentosa: Patient's fight for sight. Proc. 4th Congr Int Retinitis Pigmentosa Assoc., Bad Naheim, West
9. Lane C, Boulton M, Marshall J. Transplantation of retinal pigment epithelium using
a pars plana approach. Eye 1989;3:27-32. ![]()
10. Beauchemin ML: The fine structure of the pig's retina. Albrecht v Graefes Arch Klin Exp Ophtalmol 1974;190:27-45.
11. Simoens P, Schaepdrijver L, Lauwers H. Morphologic and clinical study of the retinal
circulation in the miniature pig. A: morphology of the retinal microvasculature.
Exp Eye Res 1992;54:965-973. ![]()
12. Michon JJ, Li Z, Shioura N, Anderson RJ, Tso MOM. A comparative study of methods
of photoreceptor morphometry. Invest Ophthalmol Vis Sci 1991;32:280-4. ![]()
13. Rapp LM, Williams TP. The role of ocular pigmentation in protecting against retinal
light damage. Vision Res 1980;20:1127-1131. ![]()
14. LaVail MM, Gorrin GM. Protection from light damage by ocular pigmentation: analysis
using experimental chimeras and translocation mice. Exp Eye Res 1987;44:877-889. ![]()
15. Organisciak DT, Wang H, Li Z, Tso MOM. The protective effect of ascorbate in retinal
light damage of rats. Invest Ophthalmol Vis Sci 1985;26:1580-1588. ![]()
16. Lam S, Tso MOM, Gurne DH. Amelioration of retinal photic injury in albinos rats
by dimethylthiourea. Arch Ophthalmol 1990;108:1751-1757. ![]()
17. Goureau O, Jeanny JC, Becquet F, Hartmann MP, Courtois Y. Protection against light-induced
retinal degeneration by an inhibitor of NO synthase. Neuroreport 1993;5:233-236. ![]()
18. Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM. Basic fibroblast
growth factor and local injury protect photoreceptors from light damage in the rat.
J Neurosci 1992;12:3554-3567. ![]()
19. LaVail M, Unoki K, Yasumura D, Matthes MT, Yancopoulos GD, Steinberg RH. Multiple
growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging
effects of constant light. Proc Natl Acad Sci USA 1992;89:11249-11253. ![]()
 Return to Molecular Vision homepage
Return to Molecular Vision homepage