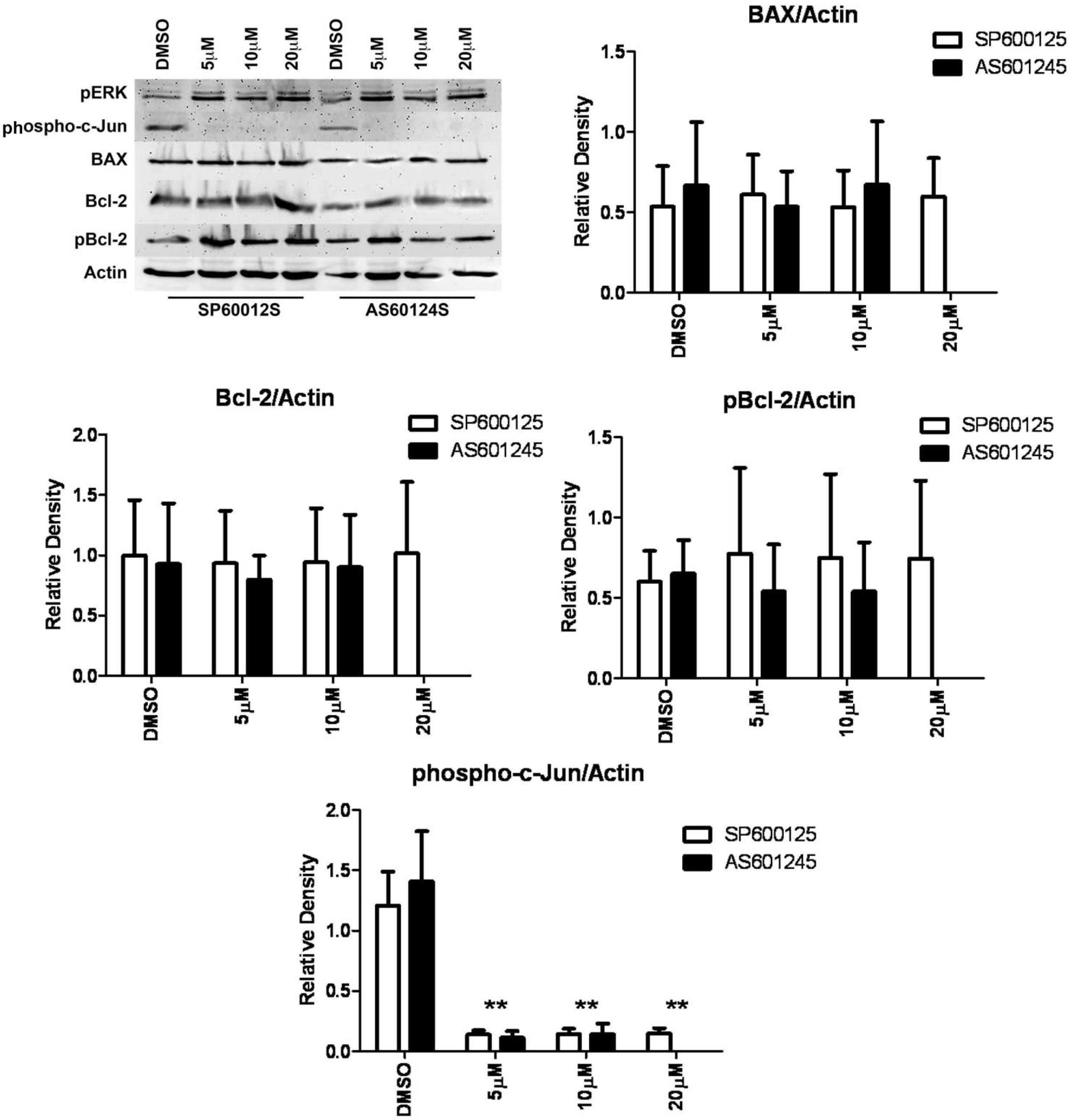
Figure 6. Western blot analysis of phospho-c-Jun in the presence of SP600125 or AS601245. HLE-B3 cell cultures were incubated for 90
min in serum-free minimal essential media (MEM), under conditions of atmospheric oxygen, containing either SP600125 (5 µM,
10 µM, and 20 µM), AS601245 (5 µM, 10 µM, and 20 µM), or DMSO (0.05%) vehicle. Cells were then exposed to hypoxia for 3 h
in the continued presence of inhibitors. At the end of the hypoxic exposure, the media were removed, and fresh, oxygenated
serum-free MEM with either inhibitor or DMSO were added to the cultures. Cells were subsequently placed in atmospheric oxygen
for 3 h. Whole cell lysates were collected at the end of the 3 h reintroduction of atmospheric oxygen. Lysates were analyzed
with western blot for phospho-c-Jun, pERK, BAX, Bcl-2, and p-BCL-2 using 25 µg of protein per lane. Anti-actin was used to
normalize the bands to ensure equivalent lane loading. The inhibition of the phosphorylation of c-Jun by either JNK inhibitor
indicates the inactivation of JNK activity, while no loss of BAX, Bcl-2, or pBcl-2 was noted. Under this condition, the phosphorylation
of ERK was unimpeded. This experiment was performed twice with two independent cell populations with identical results. Treatment
with either JNK inhibitor, SP600125 or AS601245, resulted in a marked decrease in the phosphorylation of c-Jun, indicating
that both inhibitors inhibited JNK activation. The asterisks (**) indicate p<0.01, Student
t test. BAX, Bcl-2, and pBcl-2 were not significantly diminished relative to the DMSO control, indicating that the ERK pathway,
but not the JNK pathway, is involved in the loss of Bcl-2 and pBcl-2 (refer to
Figure 5). Two experiments, using independent cell populations, were quantified using GraphPad Prism 5. Reader note: The 20 µM AS601245
was run only once as reflected in the western blot but not in the densitometry plots because we cannot generate statistics
on one run.
 Figure 6 of
Brooks, Mol Vis 2013; 19:2451-2467.
Figure 6 of
Brooks, Mol Vis 2013; 19:2451-2467.  Figure 6 of
Brooks, Mol Vis 2013; 19:2451-2467.
Figure 6 of
Brooks, Mol Vis 2013; 19:2451-2467.