Figure 1. Chemical structures of the key compounds tested in the current studies. Peptide Agonists: Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg
(BK); Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe (Des-Arg9-BK); Arg-Pro-Hyp-Gly-Phe-Ser-Pro-Phe-Arg (Hyp3-BK); Lys-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg
(Lys-BK); Met-Lys-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg (Met-Lys-BK); H-Sar-Arg-Pro-Pro-Gly-Phe-Ser-Pro-D-Phe-OH (Sar-[D-Phe9
]des-Arg9-BK); H-Arg-Pro-Hyp-Gly-Thi-Ser-Pro-4-Me-Tyrψ(CH2NH)-Arg-OH (RMP-7; CH2NH denotes a reduced peptide bond between
the 4-Me-tyrosine and arginine amino acids). Peptide Antagonist: H-D-Arg-Arg-Pro-Hyp-Gly-Thi-Ser-D-Tic-Oic-Arg-OH (HOE-140;
Icatibant). D = D configuration of amino acid; (D-BT) = (3S)[amino]-5-(carbonylmethyl)-2,3-dihydro-1,5-benzothiazepin-4(5H)-one;
Hyp = trans-4-Hydroxy-L-proline; Igl = α-(2-Indanyl)glycine; Oic = Octahydroindole-2-carboxylic acid; Thi = O-(2-thienyl)-alanine;
Tic = L-1,2,3,4-Tetrahydroisoquinoline-3-carbonyl; TFA = trifluoroacetic acid.
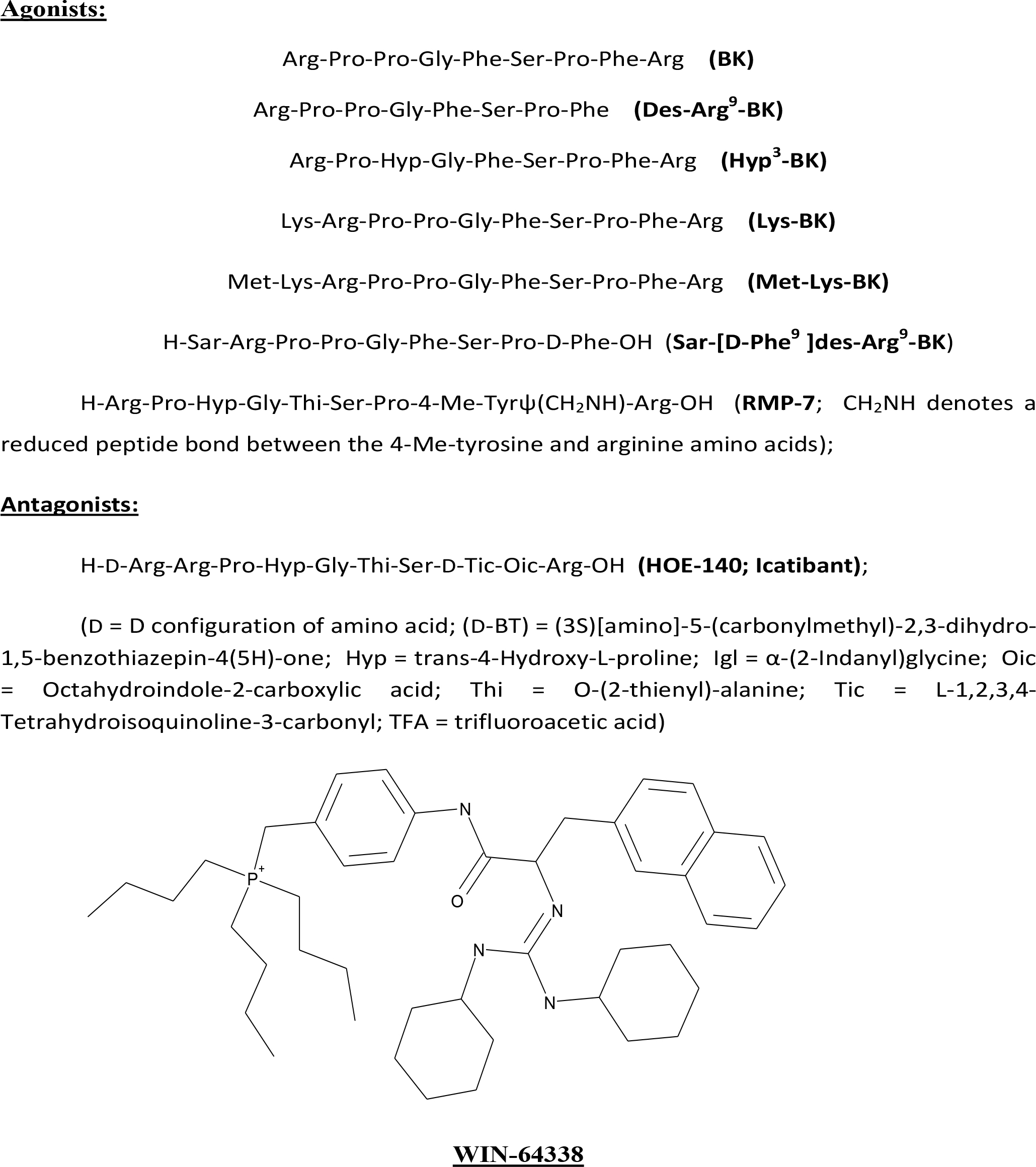
 Figure 1 of
Sharif, Mol Vis 2013; 19:1356-1370.
Figure 1 of
Sharif, Mol Vis 2013; 19:1356-1370.  Figure 1 of
Sharif, Mol Vis 2013; 19:1356-1370.
Figure 1 of
Sharif, Mol Vis 2013; 19:1356-1370. 