Figure 2. The mutant is structurally weaker and aggregates more readily. A: D26G is slightly less stable. Chemical unfolding of human gamma S-crystallin (HGSC; blue) and its mutant D26G (red), monitored
using the ratio of the fluorescence intensity at 360 nm (for the unfolded form) and 320 nm (native form), followed using the
spectrofluorimeter above. Protein concentrations were 0.1 mg/ml in 50 mM Tris buffer (pH 7.3), with 1 mM EDTA and 5 mM DTT.
B: The mutant denatures at a lower temperature. Thermal unfolding of HGSC (blue) and D26G (red), monitored by following the
change in the wavelength of emission (from 327 nm for the native form to 338 nm for the denatured form), with temperature,
using the spectrofluorimeter. Protein concentrations were 100 μg/ml in 50 mM Tris buffer (pH 7.3), with 1 mM EDTA and 5 mM
DTT. C: D26G self-aggregates. Turbidity or light scattering by wild-type (blue) and D26G HGSC (red) at 600 nm, measured as a function
of time, is shown. The temperature was fixed as 65 °C, and A600 nm was followed, using the spectrofluorimeter, using 2.5 nm excitation and emission slits. Protein concentrations were 10 µg/ml,
in 50 mM Tris buffer, pH 7.3, with 1 mM EDTA and 5 mM DTT.
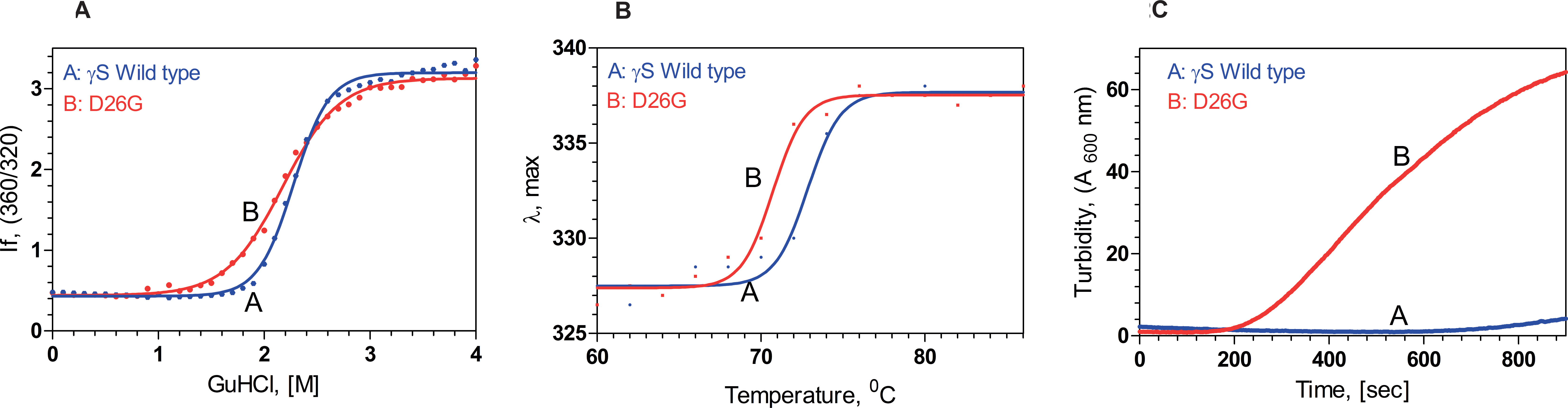
 Figure 2 of
Karri, Mol Vis 2013; 19:1231-1237.
Figure 2 of
Karri, Mol Vis 2013; 19:1231-1237.  Figure 2 of
Karri, Mol Vis 2013; 19:1231-1237.
Figure 2 of
Karri, Mol Vis 2013; 19:1231-1237. 