Figure 2. The RB1 gene and RB1 protein are expressed in RB116 cells. A: Multiplex ligation-dependent probe amplification RB1 (MLPA) is shown for RB116 cells. Gene dosage analysis shows a normal copy number (2) corresponding to all probes, with no
duplications or deletions. Probes targeted 23 of RB1’s 27 exons and three nearby genes (DLEU1, CHC1L, ITM2B). Y79 cells, with a known multiexon deletion, served as a positive control. Normal human peripheral blood served as a negative
control. B: Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) analysis shows RB1 mRNA expression in RB116 cells. RB116 cells were evaluated alongside RB1-negative RB143 cells and RB1-positive MDA-MB231
breast cancer cells. RB116 cell expression was set at 1.0. The experiment was repeated three times and the error bars show
standard deviation. No p values were calculated, as this was an experiment to determine presence or absence of RB1 and not
meant to be comparative between cell lines. RB1 mRNA (mRNA) was detected in both RB116 and MDA-MB231 cells. C: western blot analysis detects RB1 protein in RB116 cells. Western immunoblotting was performed on RB116 cells and detected
the expected p110 RB1 band that was identical to the positive controls (purified RB1 protein, MOLT4 human leukemia cells,
and U87 human glioma cells). No band was seen in the lane containing lysate from the negative control (RB1 negative RB143
cells). The blot was reprobed with alpha-tubulin to ensure equal protein loading of the cell lysates. D: Immunocytochemistry demonstrates perinuclear localization of RB1 protein in RB116 cells. RB116 cells were compared with
the negative control (Y79 cells) and the positive control (MDA-MB231 cells). The experiment was repeated three times. Note
the perinuclear localization of RB1 in RB116 cells. A control slide of RB116 cells received an isotype control antibody instead
of the anti-RB1 antibody.
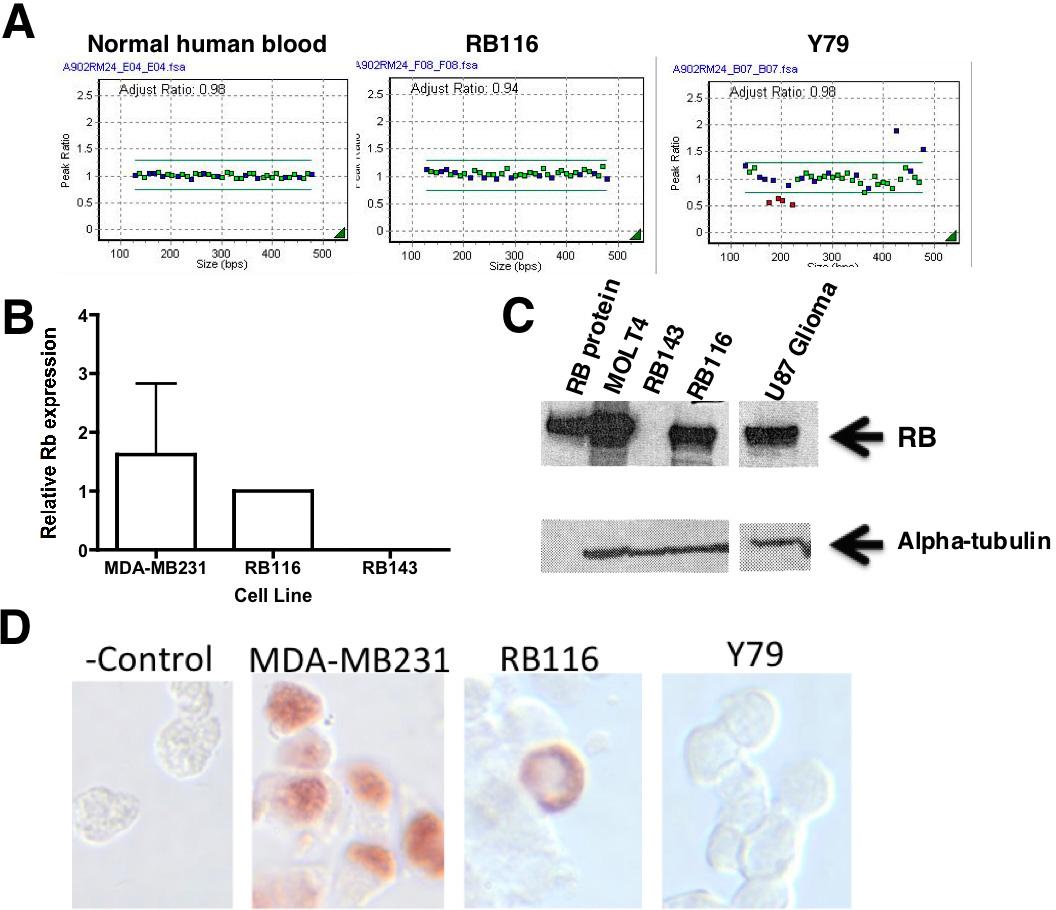
 Figure 2 of
Bejjani, Mol Vis 2012; 18:2805-2813.
Figure 2 of
Bejjani, Mol Vis 2012; 18:2805-2813.  Figure 2 of
Bejjani, Mol Vis 2012; 18:2805-2813.
Figure 2 of
Bejjani, Mol Vis 2012; 18:2805-2813. 