Figure 5. Identification of the
region in PITX2 that interacts with EFEMP2. Upper panel:
Schematic representation of full-length PITX2C, PITX2A and the
PITX2 deletion constructs used in this study. Lower Panel: The
HA-PITX2A wild type (WT) and deletion constructs (Δ39–98,
Δ99–232 and Δ233–271) were tested for interaction with EFEMP2ΔN36
using co-immunoprecipitation experiments. All PITX2 constructs
(WT, Δ39–98, Δ99–232 and Δ233–271) bound to V5- EFEMP2ΔN36
were immunoprecipitated with anti-V5 antibody and detected
subsequently by immunoblotting using anti-HA antibody. This
experiment confirms that the all PITX2 constructs used in this
experiment interact with EFEMP2ΔN36 suggesting that
the NH2-terminal region before the homeodomain in
PITX2, commonly shared by all these PITX2 constructs interacts
with EFEMP2N36. Inputs represent 20% of the cell
lysates used for immunoprecipitation experiments.
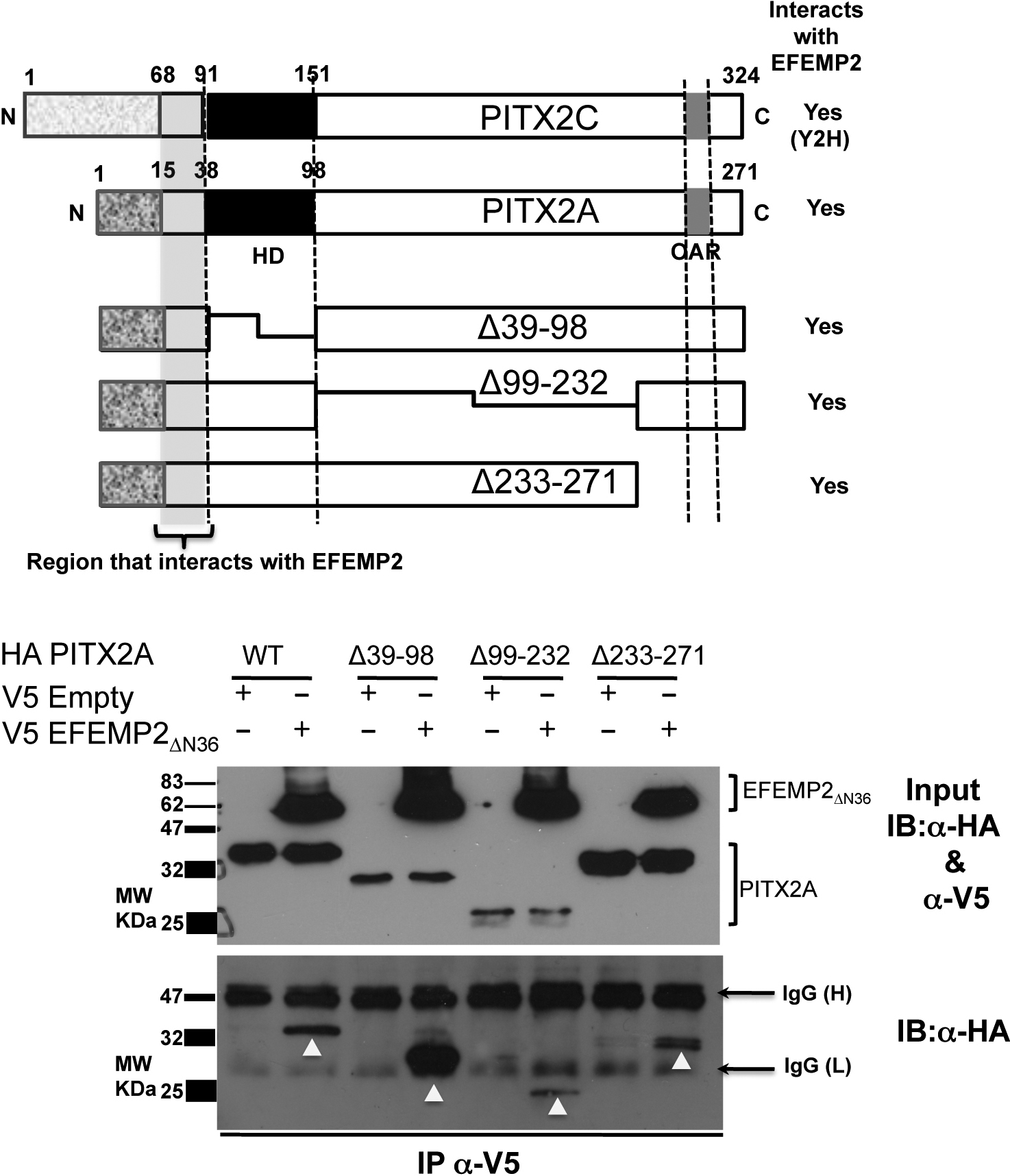
 Figure 5
of Acharya, Mol Vis 2012; 18:2182-2189.
Figure 5
of Acharya, Mol Vis 2012; 18:2182-2189.  Figure 5
of Acharya, Mol Vis 2012; 18:2182-2189.
Figure 5
of Acharya, Mol Vis 2012; 18:2182-2189. 