Figure 1. Summary of the yeast
two-hybrid analysis using PITX2A and PITX2C as “bait.” EFEMP2
was identified as a novel PITX2-interacting protein by
independent yeast two-hybrid (Y2H) analysis with two commonly
expressed PITX2 isoforms. Y2H screening with PITX2A resulted in
14 EFEMP2 cDNAs from approximately 250,000 yeast clones
while 12 EFEMP2 cDNAs were derived from screening
approximately one million yeast clones with PITX2C. Combining
all positives, the largest EFEMP2 clone lacks the NH2-terminal
36 amino acid residues while the smallest EFEMP2 clone
lacks the NH2-terminal 134 amino acid residues. A
schematic representation of EFEMP2 protein therefore shows that
the PITX2 interacting domain in EFEMP2 lies between the second
EGF-like module and the COOH-terminal fibulin-like module (135
to 443 amino acid residues).
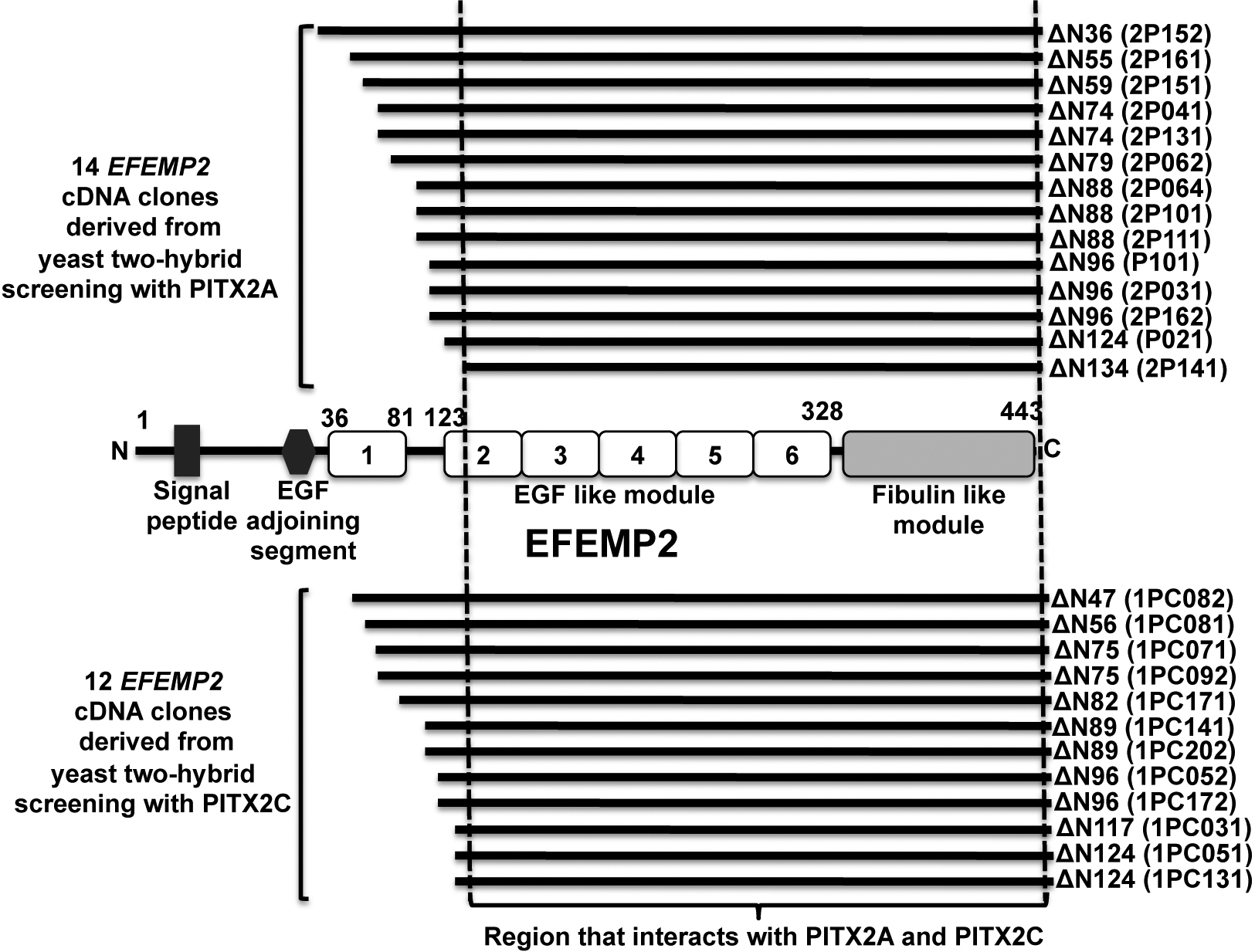
 Figure 1
of Acharya, Mol Vis 2012; 18:2182-2189.
Figure 1
of Acharya, Mol Vis 2012; 18:2182-2189.  Figure 1
of Acharya, Mol Vis 2012; 18:2182-2189.
Figure 1
of Acharya, Mol Vis 2012; 18:2182-2189. 