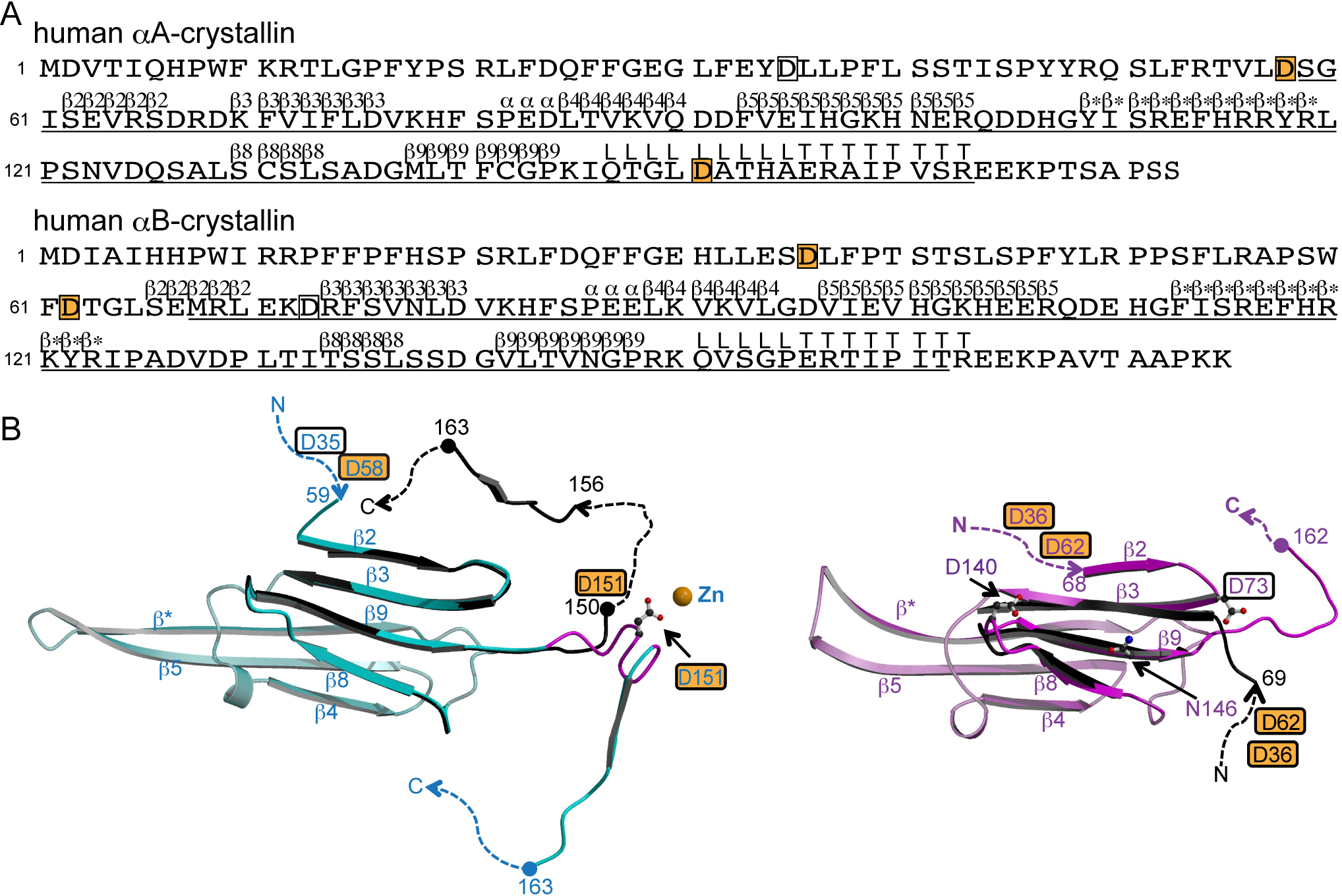
Figure 1. The structures of αA- and
αB-crystallins.
A: Primary and secondary structures of
αA- and αB-crystallins [
13].
The secondary structure is denoted by upper letters; β, α, L and
T indicate strands, helices, loops and COOH-terminal tail,
respectively. Regions for which structural information is
available in bovine αA-crystallin (PDB ID: 3L1E) and human
αB-crystallin (PDB ID: 3L1G) are underlined [
13].
The letters in boxes indicate aspartyl residues in which
digestion by Asp-N occurs, and those on an orange background
indicate isomerizable residues in human αA- and αB-crystallins.
B: Tertiary structures of αA- (left) and αB- (right)
crystallins. Numbers indicate the amino acid positions. The
dashed lines indicate disordered regions. The letters in boxes
indicate aspartyl residues in which digestion by Asp-N occurs,
and those on an orange background indicate isomerizable residues
in αA-crystallin and in αB-crystallin in vivo. Asp151 in
αA-crystallin, and Asp73, Asp140 and Asn146 in αB-crystallin
noted in the text are drawn in ball-and-stick models to make
clear their positions. The figures were drawn by
MOLSCRIPT [
23]
and
RASTER3D
[
24].
Left: The recombinant bovine αA-crystallins (cyan, PDB ID:
3L1E). The β strands 2, 3, 8 and 9, the hinge loop region noted
in the text (Gln147 - Ala155) and the C-terminal tail of the
other bovine αA-crystallin crystal structure (black, PDB ID:
3L1F) are superposed [
13].
The hinge loop region is colored in magenta in 3L1E. Right: One
of the crystal structures of recombinant human αB-crystallins
(pink, PDB ID: 3L1G). The β strands 3, 8 and 9 and available NH
2-terminal
region of the representative model in NMR structure (black,
molecule A in model 1 in PDB ID: 2KLR) are superposed [
13,
15].
 Figure 1
of Shimizu, Mol Vis 2012; 18:1823-1827.
Figure 1
of Shimizu, Mol Vis 2012; 18:1823-1827.  Figure 1
of Shimizu, Mol Vis 2012; 18:1823-1827.
Figure 1
of Shimizu, Mol Vis 2012; 18:1823-1827.