Figure 2. Functional assays of the two NTF4 mutations. A: Immunofluorescence analysis of NTF4-transfected HeLa cells. NTF4-transfected HeLa cells were fixed with 3% paraformaldehyde and stained with mouse monoclonal antibodies against FLAG. The
immunofluorescence pattern of NTF4 wildtype protein with FLAG tag suggested cytoplasmic localization. The pattern of p.Gly157Ala
variant protein showed no difference with that of wildtype. However, p.Ala182Val variant protein could not be detected despite
the indication of GFP signal for a transfected cell. Scale bar: 20 μm. B: Gene expression analysis of NTF4-transfected HeLa cells. Total RNA of the NTF4-transfected cells was collected, extracted and reverse transcribed. NTF4 and β-actin (ACTB) gene expressions were detected by polymerase chain reaction. All NTF4-transfected cells, but not empty vector-transfected cells, expressed NTF4 transcript. C: Solubility analysis of NTF4 proteins. NTF4-transfected HeLa cells were lysed by the Triton X-100 lysis buffer. The soluble supernatant and insoluble pellets were analyzed
separately by immunoblotting using horseradish peroxidase-conjugated mouse monoclonal antibodies against FLAG and β-actin.
NTF4 wildtype and p.Gly157Ala variant proteins were expressed and soluble in Triton X-100 buffer. However, p.Gly157Ala variant
protein was less soluble than wildtype. The p.Ala182Val variant protein could not be detected in both fractions. D: Hydrophobicity analysis of NTF4 proteins. The hydrophobicity of NTF4 amino acid sequences was predicted by ProtScale. Around
the position 150, the signal in p.Gly157Ala was elevated compared to that in wild type (WT), suggesting increase in hydrophobicity
of the variant.
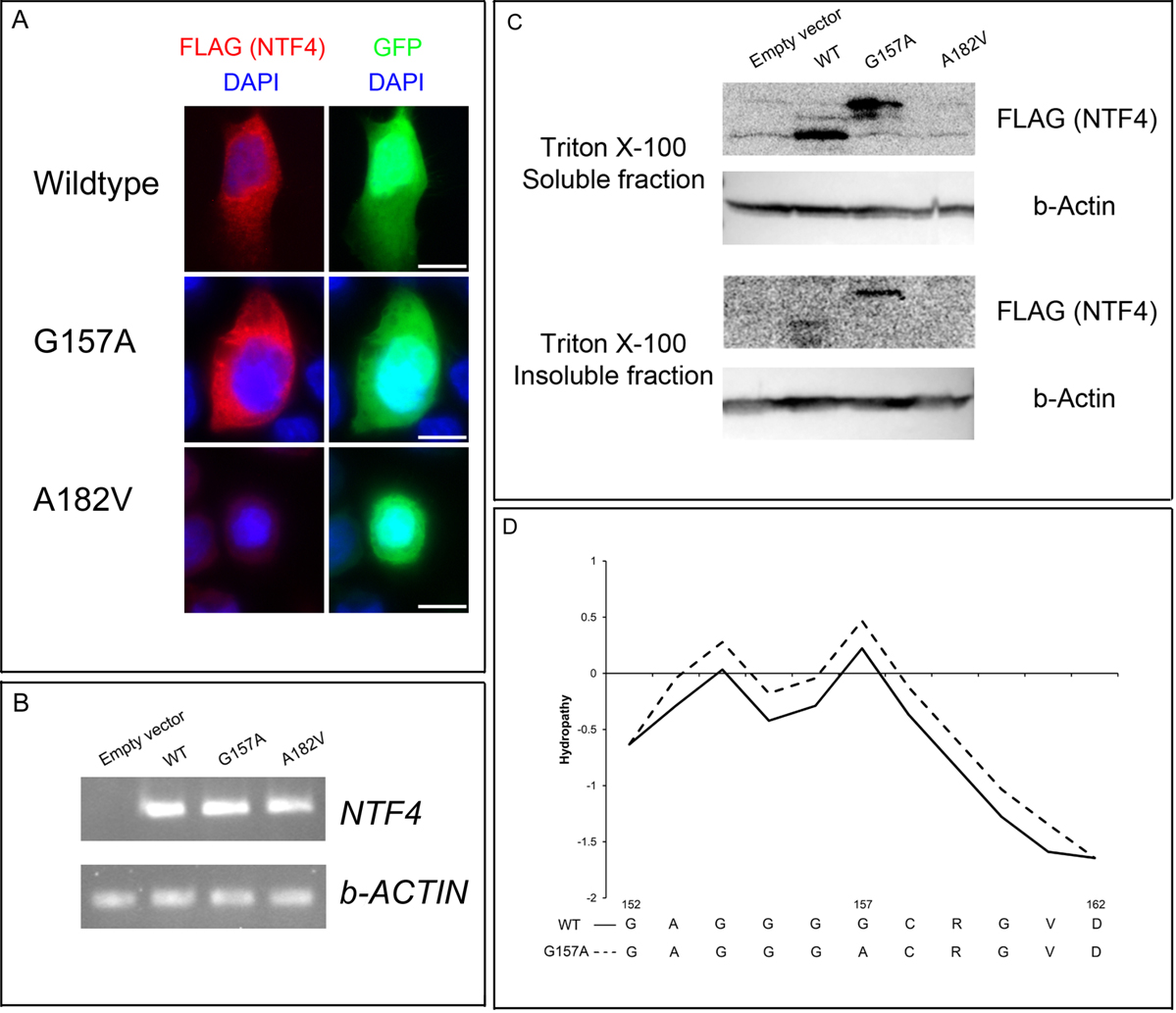
 Figure 2 of
Chen, Mol Vis 2012; 18:1763-1772.
Figure 2 of
Chen, Mol Vis 2012; 18:1763-1772.  Figure 2 of
Chen, Mol Vis 2012; 18:1763-1772.
Figure 2 of
Chen, Mol Vis 2012; 18:1763-1772. 