Figure 8. Summary diagram of the
proposed adrenomedullin (ADM) signaling pathway in the retina.
Protein kinase C (PKC) activation can lead to increased
transcription of the ADM gene via a PKC enhancer element. The
ADM precursor preproadrenomedullin is cleaved to
proadrenomedullin, which is then cleaved into the secreted
peptide ADM and the proadrenomedullin NH2-terminal
peptide (PAMP). ADM is secreted and binds to the G-protein
coupled receptor calcitonin receptor like receptor (CRLR) that
is associated with either the receptor activity modifying
protein RAMP2 or RAMP3 to activate a signaling cascade that
increases cyclic adenosine monophosphate (cAMP) by activating
adenylyl cyclase. Increases in cAMP levels activate protein
kinase A (PKA), which increases calcium levels by opening
membrane calcium channels or by releasing intracellular calcium
stores. The overall increase in intracellular calcium can
increase nitric oxide (NO) production by directly stimulating
nNOS or by activating nNOS through the activation of the
calcium-activated phosphatase, calcineurin (CaN), which
dephosphorylates nNOS at an inhibitory phosphorylation site at
serine847. Increases in NO production can then
increase in cGMP synthesis by activating soluble guanylyl
cyclase (sGC).
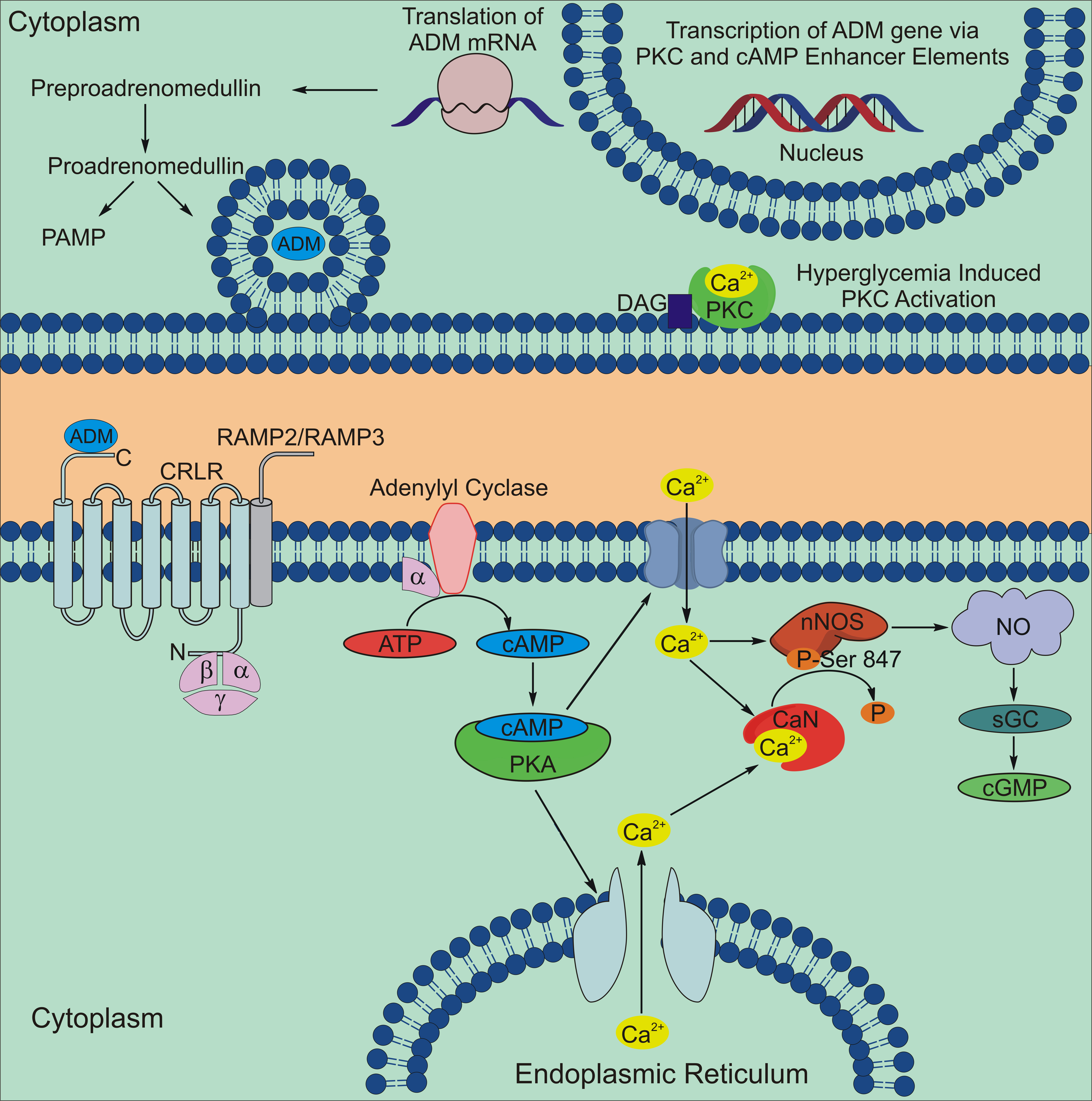
 Figure 8
of Blom, Mol Vis 2012; 18:1339-1353.
Figure 8
of Blom, Mol Vis 2012; 18:1339-1353.  Figure 8
of Blom, Mol Vis 2012; 18:1339-1353.
Figure 8
of Blom, Mol Vis 2012; 18:1339-1353. 