Figure 4. The percent distribution of phosphorylation sites of phosphorylated crystallin proteins in normal human lens proteins. Distribution
of in vivo phosphorylation sites in A: βB1-crystallin; B: βB2-crystallin; C: αB-crystallin; D: γD -crystallin; E: αA-crystallin; and F: βS-crystallin. Ser-81 (31%) and Ser93/Thr-118 (25%) are the predominant phosphorylation-sites in βB1-crystallin and βB2-crystallin,
respectively. In addition, the phosphorylation of αB-crystallin was shown to distribute almost evenly over the whole crystallin
molecule at Ser-19 (23%), Ser-21 (22%), Ser-59 (22%), and Ser-139 (22%). In contrast, there was at least one predominant phosphorylated
site in other crystallin proteins, i.e., Ser-75 (50%) in γD-crystallin, Thr-148 (33%) in αA-crystallin, and Tyr-11/Ser-167
(33%) in βS-crystallin.
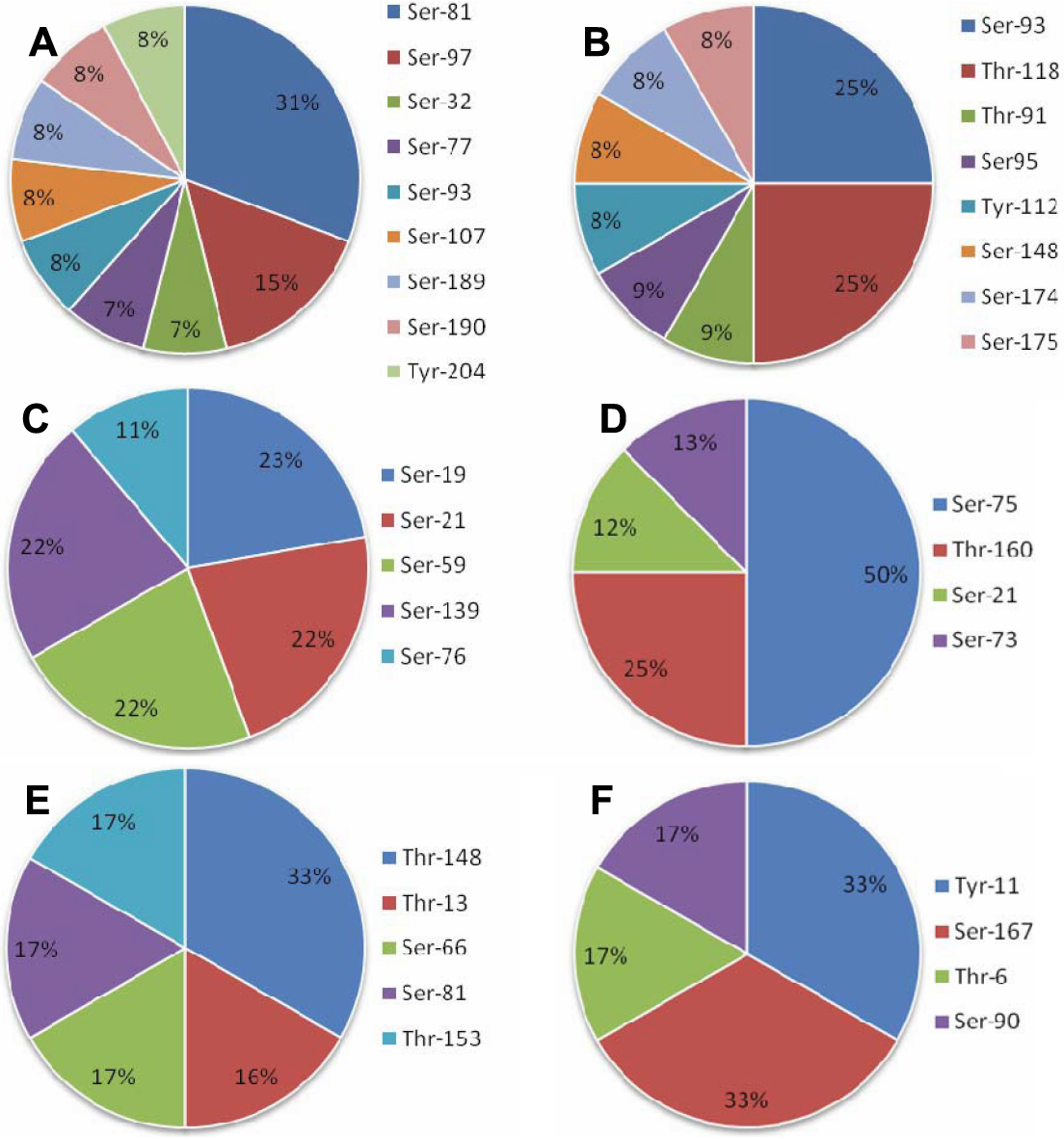
 Figure 4 of
Huang, Mol Vis 2011; 17:186-198.
Figure 4 of
Huang, Mol Vis 2011; 17:186-198.  Figure 4 of
Huang, Mol Vis 2011; 17:186-198.
Figure 4 of
Huang, Mol Vis 2011; 17:186-198. 