Figure 9. Cell signaling underlying the regulation of actomyosin contraction. A: MLC phosphorylation: MLC kinase (MLCK) drives phosphorylation and MLC phosphatase (MLCP) induces dephosphorylation. MLCP
is a trimeric complex consisting of a phosphatase (PP1cδ), a myosin-binding subunit (MYPT1), and a subunit of unknown function
(M20). MLCP activity is regulated by kinases like ROCK and integrin-linked kinase (ILK) through phosphorylation of MYPT1 at
specific sites. This inactivates the catalytic subunit, thus preventing MLC dephosphorylation. B: Autoinhibition of MLCP: The substrate site of PP1cδ is accessible when neither Thr696 nor Thr853 is phosphorylated. However,
when MYPT1 is phosphorylated at Thr696 or Thr853, the phosphorylated residues interact with the active site of PP1cδ and suppress
the phosphatase activity. While both ROCK and ILK are known to phosphorylate Thr696, phosphorylation at Thr853 seems to be
regulated exclusively by ROCK.
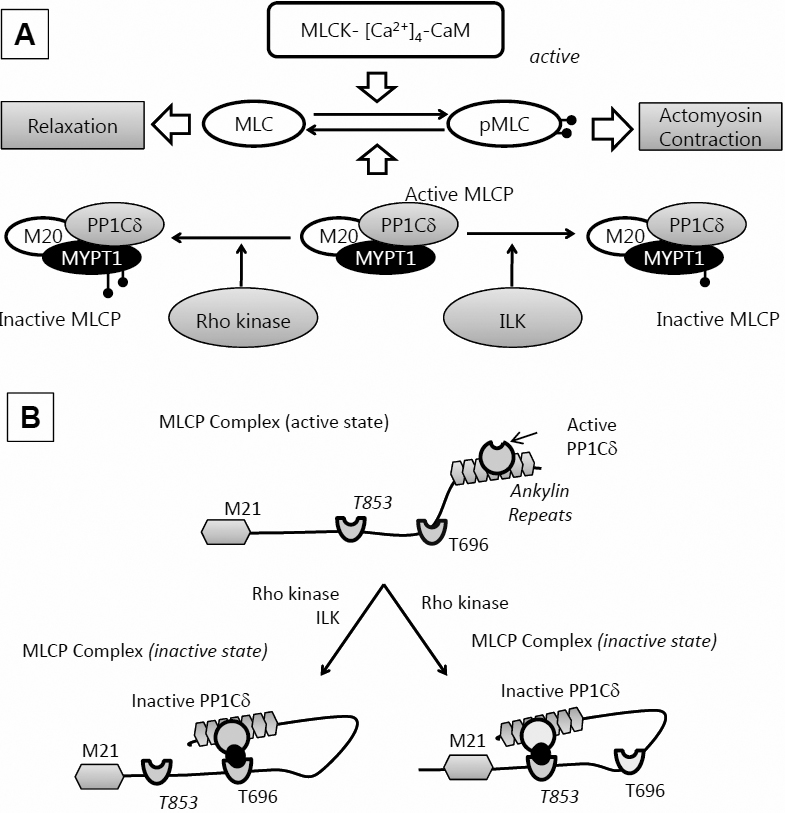
 Figure 9 of
Ramachandran, Mol Vis 2011; 17:1877-1890.
Figure 9 of
Ramachandran, Mol Vis 2011; 17:1877-1890.  Figure 9 of
Ramachandran, Mol Vis 2011; 17:1877-1890.
Figure 9 of
Ramachandran, Mol Vis 2011; 17:1877-1890. 